Spinosad
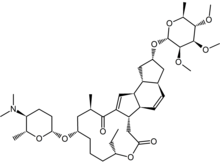 Spinosyn A
| |
 Spinosyn D
| |
| Identifiers | |
|---|---|
| |
| ChEBI |
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| |
| Properties | |
| C41H65NO10 (A) C42H67NO10 (D) | |
| Pharmacology | |
| QP53BX03 (WHO) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Spinosad is an insecticide and medication. It is used to treat head lice.
It is in a medication class called pediculicides.[1]
It was originally made from the bacterial species Saccharopolyspora spinosa. The genus Saccharopolyspora was discovered in 1985 in crushed sugarcane.
Use
Spinosad has been used to control of a variety of insect, including Lepidoptera, Diptera, Thysanoptera, Coleoptera, Orthoptera, and Hymenoptera.[2] It was first registered as a pesticide in the United States for use on crops in 1997.[2] Its labeled use rate is set at 1 ppm (1 mg a.i./kg of grain) and its maximum residue limit (MRL) or tolerance is set at 1.5 ppm. Spinosad's widespread commercial launch was deferred, awaiting final MRL or tolerance approvals in a few remaining grain-importing countries. It is considered a natural product, thus is approved for use in organic agriculture by numerous nations.[3] Two other uses for spinosad are for pets and humans. Spinosad has recently been used in oral preparations (as Comfortis) to treat C. felis, the cat flea, in canines and felines; the optimal dose set for canines is reported to be 30 mg/kg.[4]
Medical
This product can also be used for medicinal purposes as it is used to treat head lice (spinosad suspension) and is in a medication class called pediculicides.[1]
Safety
Spinosad has high efficacy, a broad insect pest spectrum, low mammalian toxicity, and a good environmental profile, a unique feature of the insecticide compared to others currently used for the protection of grain products.[3] It is regarded as natural product-based, and approved for use in organic agriculture by numerous national and international certifications.[2] Spinosad residues are highly stable on grains stored in bins, with protection ranging from 6 months to 2 years.[3][clarification needed] Ecotoxicology parameters have been reported for spinosad, and are:[5]
- in rat (Rattus norvegicus Bergenhout, 1769), acute oral: LD50 >5000 mg/kg (nontoxic)
- in rat (R. norvegicus), acute dermal: LD50 >2000 mg/kg (nontoxic)
- in California quail (Callipepla californica Shaw, 1798), oral toxicity: LD50 >2000 mg/kg (nontoxic)
- in duck (Anas platyrhynchos domestica Linnaeus, 1758), dietary toxicity: LC50 >5000 mg/kg (nontoxic)
- in rainbow trout (Oncorhynchus mykiss Walbaum, 1792), LC50-96h = 30.0 mg/l (slightly toxic)
- in Honeybee (Apis mellifera Linnaeus, 1758), LD50 = 0.0025 mg/bee (highly toxic if directly sprayed on and of dried residues).
Chronic exposure studies failed to induce tumor formation in rats and mice; mice given up to 51 mg/kg/day for 18 months resulted in no tumor formation.[6] Similarly, administration of 25 mg/kg/day to rats for 24 months did not result in tumor formation.[7]
Mechanism of action
Spinosad is highly active, by both contact and ingestion, in numerous insect species.[3] Its overall protective effect varies with insect species and life stage. It affects certain species only in the adult stage, but can affect other species at more than one life stage. The species subject to very high rates of mortality as larvae, but not as adults, may gradually be controlled through sustained larval mortality.[3] The mode of action of spinosoid insecticides is by a neural mechanism.[8] The spinosyns and spinosoids have a novel mode of action, primarily targeting binding sites on nicotinic acetylcholine receptors (nAChRs) of the insect nervous system that are distinct from those at which other insecticides have their activity. Spinosoid binding leads to disruption of acetylcholine neurotransmission.[4] Spinosad also has secondary effects as a γ-amino-butyric acid (GABA) neurotransmitter agonist.[4] It kills insects by hyperexcitation of the insect nervous system.[4] Spinosad so far has proven not to cause cross-resistance to any other known insecticide.[9]
Spinosyn A
Spinosyn A does not appear to interact directly with known insecticidal-relevant target sites, but rather acts via a novel mechanism.[8] Spinosyn A resembles a GABA antagonist and is comparable to the effect of avermectin on insect neurons.[10] Spinosyn A is highly active against neonate larvae of the tobacco budworm, Heliothis virescens, and is slightly more biologically active than spinosyn D. In general, spinosyns possessing a methyl group at C6 (spinosyn D-related analogs) tend to be more active and less affected by changes in the rest of the molecule.[9] Spinosyn A is slow to penetrate to the internal fluids of larvae; it is also poorly metabolized once it enters the insect.[9] The apparent lack of spinosyn A metabolism may contribute to its high level of activity, and may compensate for the slow rate of penetration.[9]
Chemistry
The bacteria produce yellowish-pink aerial hyphae, with bead-like chains of spores enclosed in a characteristic hairy sheath.[11] This genus is defined as aerobic, Gram-positive, nonacid-fast actinomycetes with fragmenting substrate mycelium. S. spinosa was isolated from soil collected inside a nonoperational sugar mill rum still in the Virgin Islands. Spinosad is a mixture of chemical compounds in the spinosyn family that has a generalized structure consisting of a unique tetracyclic ring system attached to an amino sugar (D-forosamine) and a neutral sugar (tri-Ο-methyl-L-rhamnose).[4] Spinosad is relatively nonpolar and not easily dissolved in water.[12] Spinosad is an insecticide derived from a family of natural products obtained by fermentation of S. spinosa. Spinosyns occur in over 20 natural forms, and over 200 synthetic forms (spinosoids) have been produced in the lab.[10] Spinosad contains a mix of two spinosoids, spinosyn A, the major component, and spinosyn D (the minor component), in a roughly 17:3 ratio.[11]
Society and culture
Cost
The cost of this medication in the U.S. is $232 (USD) for 120 ml topical suspension 0.9% [13]
Names
Spinosad is sold under the trade names, Comfortis, Trifexis, and Natroba.[14][15] Trifexis also includes milbemycin oxime. Comfortis and Trifexis brands treat adult fleas on pets; the latter also prevents heartworm disease. Natroba is sold for treatment of human head lice. Spinosad is also commonly used to kill thrips.[16][17][18]
References
- ↑ 1.0 1.1 "Spinosad Topical: MedlinePlus Drug Information". medlineplus.gov. Archived from the original on 11 April 2021. Retrieved 8 April 2021.
- ↑ 2.0 2.1 2.2 Sparks, Thomas; James E. Dripps; Gerald B Watson; Doris Paroonagian (6 November 2012). "Resistance and cross-resistance to the spinosyns- A review and analysis". Pesticide Biochemistry and Physiology. 102: 1–10. doi:10.1016/j.pestbp.2011.11.004. Archived from the original on 1 November 2003. Retrieved 17 November 2011.
- ↑ 3.0 3.1 3.2 3.3 3.4 Hertlein, Mark; Gary D. Thompson; Bhadriraju Subramanyam; Christos G. Athanassiou (12 January 2011). "Spinosad: A new natural product for stored grain protection". Stored Products. 47 (3): 131–146. doi:10.1016/j.jspr.2011.01.004. Archived from the original on 29 August 2021. Retrieved 3 May 2012.
- ↑ 4.0 4.1 4.2 4.3 4.4 Qiao, Meihua; Daniel E. Snyder; Jeffery Meyer; Alan G. Zimmerman; Meihau Qiao; Sonya J. Gissendanner; Larry R. Cruthers; Robyn L. Slone; Davide R. Young (12 September 2007). "Preliminary Studies on the effectiveness of the novel pulicide, spinosad, for the treatment and control of fleas on dogs". Veterinary Parasitology. 150 (4): 345–351. doi:10.1016/j.vetpar.2007.09.011. PMID 17980490.
- ↑ "Codling Moth and Leafroller Control Using Chemicals" (PDF). Entomology.tfrec.wsu.edu. Archived from the original (PDF) on 2016-03-07. Retrieved 2012-10-20.
- ↑ Stebbins, K. E. (2002). "Spinosad Insecticide: Subchronic and Chronic Toxicity and Lack of Carcinogenicity in CD-1 Mice". Toxicological Sciences. 65 (2): 276–287. doi:10.1093/toxsci/65.2.276. PMID 11812932. Archived from the original on 2015-07-23. Retrieved 2015-03-08.
- ↑ Yano, B. L. (2002). "Spinosad Insecticide: Subchronic and Chronic Toxicity and Lack of Carcinogenicity in Fischer 344 Rats". Toxicological Sciences. 65 (2): 288–298. doi:10.1093/toxsci/65.2.288. PMID 11812933. Archived from the original on 2020-04-08. Retrieved 2015-03-08.
- ↑ 8.0 8.1 Orr, Nailah; Andrew J. Shaffner; Kimberly Richey; Gary D. Crouse (30 April 2009). "Novel mode of action of spinosad: Receptor binding studies demonstrating lack of interaction with known insecticidal target sites". Pesticide Biochemistry and Physiology. 95: 1–5. doi:10.1016/j.pestbp.2009.04.009.
- ↑ 9.0 9.1 9.2 9.3 Sparks, Thomas; Gary D crouse; Gregory Durst (30 March 2001). "Natural products as insecticides: the biology, biochemistry and quantitative structure-activity relationships of spinosyns and spinosoids". Pest Manag Sci. 57 (10): 896–905. doi:10.1002/ps.358. PMID 11695182.
- ↑ 10.0 10.1 Watson, Gerald (31 May 2001). "Actions of Insecticidal Spinosyns on gama-Aminobutyric Acid Responses for Small-Diameter Cockroach Neurons". Pesticide Biochemistry and Physiology. 71: 20–28. doi:10.1006/pest.2001.2559.
- ↑ 11.0 11.1 Mertz, Frederick; Raymond C. Yao (Jan 1990). "Saccharopolyspora spinosa sp. nov. Isolated from soil Collected in a Sugar Mill Rum Still". International Journal of Systematic Bacteriology. 40 (1): 34–39. doi:10.1099/00207713-40-1-34.
- ↑ Crouse, Gary; Thomas C Sparks; Joseph Schoonover; James Gifford; James Dripps; Tim Brue; Larry L Larson; Joseph Garlich; Chris Hatton; Rober L Hill; Thomas V Worden; Jacek G Martynow (27 September 2000). "Recent advances in the chemistry of spinosyns". Pest Manag Sci. 57 (2): 177–185. doi:10.1002/1526-4998(200102)57:2<177::AID-PS281>3.0.CO;2-Z. PMID 11455648.
- ↑ "Spinosad topical Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 8 September 2015. Retrieved 8 April 2021.
- ↑ "Spinosad international brands". Drugs.com. 3 January 2020. Archived from the original on 20 October 2012. Retrieved 30 January 2020.
- ↑ "Spinosad US brands". Drugs.com. 3 January 2020. Archived from the original on 20 October 2020. Retrieved 30 January 2020.
- ↑ "Spinosad - brand name list from". Drugs.com. Archived from the original on 2020-10-20. Retrieved 2012-10-20.
- ↑ "UC Davis School of Vet Med". Vetmed.ucdavis.edu. Archived from the original on 2015-03-06. Retrieved 2012-10-20.
- ↑ "Safer Flea Control | Insects in the City". Citybugs.tamu.edu. Archived from the original on 2013-05-15. Retrieved 2012-10-20.
Further reading
- The non‐target impact of spinosyns on beneficial arthropods Archived 2019-06-05 at the Wayback Machine
- Spinosad toxicity to pollinators and associated risk Archived 2019-03-28 at the Wayback Machine
External links
- "Spinosad". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 2020-10-21. Retrieved 2020-01-30.
- Monograph
- Pages with script errors
- Chemical articles with multiple compound IDs
- Multiple chemicals in an infobox that need indexing
- Chemical articles with multiple CAS registry numbers
- Chemical articles with multiple PubChem CIDs
- Chemical articles with multiple ChEBIs
- Articles without KEGG source
- Articles without UNII source
- Chembox CAS registry number linked
- Pages using collapsible list with both background and text-align in titlestyle
- Articles containing unverified chemical infoboxes
- Wikipedia articles needing clarification from June 2014
- Articles with invalid date parameter in template
- Webarchive template wayback links
- Biological pest control
- Biopesticides
- Insecticides
- Organic gardening
- Rhamnosides
- Sustainable agriculture