Delavirdine
 | |
 | |
| Names | |
|---|---|
| Pronunciation | del a' vir deen[1] |
| Trade names | Rescriptor |
| Other names | Delavirdine mesylate |
| |
| Clinical data | |
| Drug class | Non-nucleoside reverse transcriptase inhibitor (NNRTI)[2] |
| Main uses | HIV/AIDS[2] |
| Side effects | Tiredness, dizziness, headache, rash[1] |
| Pregnancy category |
|
| Routes of use | By mouth |
| Typical dose | 400 mg TID[2] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a600034 |
| Legal | |
| Legal status |
|
| Pharmacokinetics | |
| Bioavailability | 85% |
| Protein binding | 98% |
| Metabolism | Liver (CYP3A4- and CYP2D6-mediated) |
| Elimination half-life | 5.8 hours |
| Excretion | Kidney (51%) and fecal (44%) |
| Chemical and physical data | |
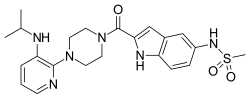
| Formula | C22H28N6O3S |
| Molar mass | 456.57 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Delavirdine (DLV), sold under the brand name Rescriptor, is a medication used to treat HIV/AIDS.[2] It is used together with other HIV medicines; though is not a preferred treatment.[2] It is taken by mouth, three times per day.[2]
Common side effects include tiredness, dizziness, headache, and rash.[1] Other symptoms may include Stevens-Johnson syndrome, central obesity, and immune reconstitution syndrome.[2] Safety in pregnancy is unclear.[2] It is a non-nucleoside reverse transcriptase inhibitor (NNRTI).[2]
Delavirdine was approved for medical use in the United States in 1997.[2] It has been discontinued in the United States as of 2021.[2] It is not commonly used.[1]
Medical uses
Its efficacy is lower than other NNRTIs, especially efavirenz, and it also has an inconvenient schedule. These factors have led the U.S. DHHS not to recommend its use as part of initial therapy.[3]
The risk of cross-resistance across the NNRTI class, as well as its complex set of drug interactions, make the place of delavirdine in second-line and salvage therapy unclear, and it is currently rarely used.
Dosage
The recommended dosage is 400 mg, three times a day.[2]
Side effects
The most common side event is moderate to severe rash, which occurs in up to 20% of patients.[4] Other common adverse events include fatigue, headache and nausea. Liver toxicity has also been reported.
Interactions
Like ritonavir, delavirdine is an inhibitor of cytochrome P450 isozyme CYP3A4, and interacts with many medications. It should not be administered with a wide range of drugs, including amprenavir, fosamprenavir, simvastatin, lovastatin, rifampin, rifabutin, rifapentine, St John's wort, astemizole, midazolam, triazolam, ergot medications, and several medications for acid reflux.[3]
Synthesis
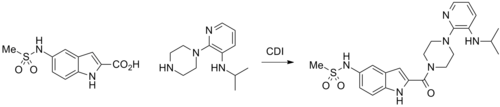
Modification of the scheme that was done for ateviridine q.v. by performing the reductive alkylation with acetone gives 2 after removal of the protecting group. Acylation of this amine with the imidazolide from 5-Methylsulfonaminoindole-2-carboxylic acid (1) affords the amide, reverse transcriptase inhibitor, atevirdine.
References
- ↑ 1.0 1.1 1.2 1.3 "Delavirdine". LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. National Institute of Diabetes and Digestive and Kidney Diseases. 2012. Archived from the original on 6 May 2021. Retrieved 23 December 2021.
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 "Delavirdine Mesylate Monograph for Professionals". Drugs.com. Archived from the original on 15 May 2016. Retrieved 23 December 2021.
- ↑ 3.0 3.1 DHHS panel. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents (May 4, 2006). (Available for download from AIDSInfo Archived 2006-05-06 at the Wayback Machine)
- ↑ "RESCRIPTOR brand of delavirdine mesylate tablets. Product information" (PDF). Archived from the original (PDF) on 2006-04-15. Retrieved 2006-05-18.
- ↑ WO 9109849, Romero DL, Mitchell MA, Thomas RC, Palmer JR, Tarpley WG, Aristoff PA, Smith HW, "Diaromatic Substituted Anti-AIDS Compounds", published 11 July 1991, assigned to Upjohn Company
- ↑ US 5563142, Palmer JR, Romero DL, Aristoff PA, Thomas RC, Smith HW, "Diaromatic substituted compounds as anti-HIV-1 agents", published 8 October 1996, assigned to Upjohn Company
- ↑ Romero DL, Morge RA, Genin MJ, Biles C, Busso M, Resnick L, et al. (May 1993). "Bis(heteroaryl)piperazine (BHAP) reverse transcriptase inhibitors: structure-activity relationships of novel substituted indole analogues and the identification of 1-[(5-methanesulfonamido-1H-indol-2-yl)-carbonyl]-4-[3- [(1-methylethyl)amino]-pyridinyl]piperazine monomethanesulfonate (U-90152S), a second-generation clinical candidate". Journal of Medicinal Chemistry. 36 (10): 1505–8. doi:10.1021/jm00062a027. PMID 7684450.
External links
| External sites: | |
|---|---|
| Identifiers: |
|
- Pages using duplicate arguments in template calls
- Webarchive template wayback links
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without UNII source
- Drugs missing an ATC code
- Portal templates with all redlinked portals
- Hepatotoxins
- Non-nucleoside reverse transcriptase inhibitors
- Pfizer brands
- Aminopyridines
- Piperazines
- Carboxamides
- Indoles
- Sulfonamides
- RTT