Maraviroc
 | |
 | |
| Names | |
|---|---|
| Pronunciation | /məˈrævɪrɒk/ mə-RAV-i-rok Selzentry: /sɛlˈzɛntri/ sel-ZEN-tree |
| Trade names | Selzentry, Celsentri |
| Other names | UK-427857, 4,4-Difluoro-N-[(1S)-3-{(1R,3s,5S)-3-[3-methyl-5-(propan-2-yl)-4H-1,2,4-triazol-4-yl]-8-azabicyclo[3.2.1]octan-8-yl}-1-phenylpropyl] cyclohexanecarboxamide |
| |
| Clinical data | |
| Drug class | CCR5 receptor antagonist[1] |
| Main uses | Treat and prevent HIV/AIDS[2] |
| Side effects | Cough, fever, rash, upper respiratory tract infection[2] |
| Pregnancy category |
|
| Routes of use | By mouth (tablets, oral solution) |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a607076 |
| Legal | |
| License data | |
| Legal status | |
| Pharmacokinetics | |
| Bioavailability | 23%[4] |
| Protein binding | ~76%[5] |
| Metabolism | Liver (CYP, predominantly CYP3A)[5] |
| Metabolites | Secondary amine formed by N-dealkylation (major) |
| Elimination half-life | 14–18 hours[5] (mean 16 hours)[6] |
| Excretion | Feces (76%), urine (20%)[5] |
| Chemical and physical data | |
| Formula | C29H41F2N5O |
| Molar mass | 513.678 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Maraviroc, sold under the brand names Selzentry and Celsentri, is an antiretroviral medication used to treat and prevent HIV/AIDS.[2] It is generally only recommended in cases which have CCR5 tropism and is not usually an initial treatment.[2] It is taken by mouth.[2]
Common side effects include cough, fever, rash, and upper respiratory tract infection.[2] Other side effects may include autoimmune disorders, allergic reactions, osteonecrosis, and liver problems.[2][1] Use is not typically recommended in pregnancy.[2] It should not be used in people with significant kidney problems.[2] It is in the CCR5 receptor antagonist and works by preventing HIV from entering cells.[1][7]
Maraviroc was approved for medical use in the United States and Europe in 2007.[2][7] In the United Kingdom it costs the NHS about £520 per month as of 2021.[1] This amount in the United States costs about 1,500 USD.[8]
Medical uses
Two randomized, placebo-controlled clinical trials, compared 209 people receiving optimized therapy plus a placebo to 426 people receiving optimized therapy plus 150 mg maraviroc once daily and 414 patients receiving optimized therapy plus 150 mg maraviroc twice daily. At 48 weeks, 55% of participants receiving maraviroc once daily and 60% of participants receiving the drug twice daily achieved a viral load of less than 400 copies/mL compared with 26% of those taking placebo; about 44% of the once-daily and 45% of the twice-daily maraviroc group had a viral load of less than 50 copies/mL compared with about 23% of those who received placebo. In addition, those who received the entry inhibitor had a mean increase in CD4+ cells of 110 cells/μL in the once-daily group, 106 cells/μL in the twice-daily group, and 56 cells/μL in the placebo group.[9][10][11]
Dosage
It is taken at a dose of 300 mg twice per day.[1]
Side effects
Maraviroc can cause serious, life-threatening side effects. These include liver problems, skin reactions, and allergic reactions. An allergic reaction may happen before liver problems occur.[12] Official labeling of Selzentry has black box warning for hepatotoxicity.[5] The MOTIVATE trials showed no clinically relevant differences in safety between the maraviroc and placebo groups.[9]
Mechanism of action
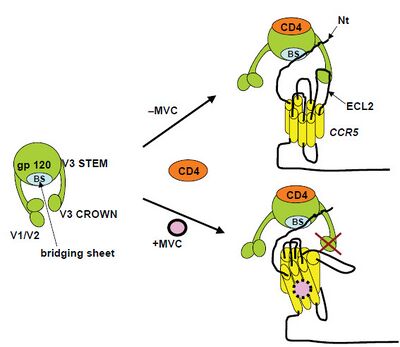
Maraviroc is an entry inhibitor. Specifically, maraviroc is a negative allosteric modulator of the CCR5 receptor, which is found on the surface of certain human cells. The chemokine receptor CCR5 is an essential co-receptor for most HIV strains and necessary for the entry process of the virus into the host cell. The drug binds to CCR5, thereby blocking the HIV protein gp120 from associating with the receptor. HIV is then unable to enter human macrophages and T cells.[14] Because HIV can also use other coreceptors, such as CXCR4, an HIV tropism test such as a trofile assay must be performed to determine if the drug will be effective.[15]
History
Maraviroc, originally designated UK-427857, was developed by the drug company Pfizer in its UK labs located in Sandwich. On April 24, 2007 the U.S. Food and Drug Administration advisory panel reviewing maraviroc's New Drug Application unanimously recommended approval for the new drug,[16] and the drug received full FDA approval on August 6, 2007 for use in treatment experienced patients.[17]
Maraviroc was approved for medical use in the European Union in September 2007.[3]
Names
Maraviroc is the International nonproprietary name (INN).[18]
Research
It also appeared to reduce graft-versus-host disease in patients treated with allogeneic bone marrow transplantation for leukemia, in a Phase I/II study.[19][20]
See also
References
- ↑ 1.0 1.1 1.2 1.3 1.4 BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 699. ISBN 978-0857114105.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 2.8 2.9 "Maraviroc Monograph for Professionals". Drugs.com. Archived from the original on 26 May 2016. Retrieved 14 November 2021.
- ↑ 3.0 3.1 "Celsentri EPAR". European Medicines Agency (EMA). Archived from the original on 12 November 2020. Retrieved 31 July 2020.
- ↑ Abel S, Russell D, Whitlock LA, Ridgway CE, Nedderman AN, Walker DK (April 2008). "Assessment of the absorption, metabolism and absolute bioavailability of maraviroc in healthy male subjects". British Journal of Clinical Pharmacology. 65 (Suppl 1): 60–7. doi:10.1111/j.1365-2125.2008.03137.x. PMC 2311408. PMID 18333867.
- ↑ 5.0 5.1 5.2 5.3 5.4 "Selzentry- maraviroc tablet, film coated Selzentry- maraviroc solution". DailyMed. 18 July 2018. Archived from the original on 25 March 2021. Retrieved 31 July 2020.
- ↑ Abel S, Back DJ, Vourvahis M (2009). "Maraviroc: pharmacokinetics and drug interactions". Antiviral Therapy. 14 (5): 607–18. PMID 19704163.
- ↑ 7.0 7.1 "Celsentri". Archived from the original on 12 November 2020. Retrieved 14 November 2021.
- ↑ "Maraviroc Prices, Coupons & Savings Tips - GoodRx". GoodRx. Archived from the original on 23 July 2021. Retrieved 14 November 2021.
- ↑ 9.0 9.1 Stephenson J (April 2007). "Researchers buoyed by novel HIV drugs: will expand drug arsenal against resistant virus". JAMA. 297 (14): 1535–6. doi:10.1001/jama.297.14.1535. PMID 17426263.
- ↑ Emmelkamp JM, Rockstroh JK (October 2007). "CCR5 antagonists: comparison of efficacy, side effects, pharmacokinetics and interactions--review of the literature". European Journal of Medical Research. 12 (9): 409–17. PMID 17933722.
- ↑ "Maraviroc reduces viral load in naive patients at 48 weeks". AIDS Patient Care and STDs. 21 (9): 703–4. September 2007. PMID 17941136.
- ↑ "Maraviroc (HIV treatment) Dosage, Side Effects". AIDSinfo. Archived from the original on 2020-02-20. Retrieved 2021-07-02.
- ↑ Latinovic, Olga; Kuruppu, Janaki; Davis, Charles; Le, Nhut; Heredia, Alonso (January 2009). "Pharmacotherapy of HIV-1 Infection: Focus on CCR5 Antagonist Maraviroc". Clinical Medicine. Therapeutics. 1: CMT.S2365. doi:10.4137/CMT.S2365. ISSN 1179-1713.
- ↑ Levy JA (January 2009). "HIV pathogenesis: 25 years of progress and persistent challenges". AIDS. 23 (2): 147–60. doi:10.1097/QAD.0b013e3283217f9f. PMID 19098484. S2CID 10571856.
- ↑ Biswas P, Tambussi G, Lazzarin A (May 2007). "Access denied? The status of co-receptor inhibition to counter HIV entry". Expert Opinion on Pharmacotherapy. 8 (7): 923–33. doi:10.1517/14656566.8.7.923. PMID 17472538. S2CID 32675897.
- ↑ "Gay News From 365Gay.com". Archived from the original on 2008-06-29. Retrieved 2021-07-02.
- ↑ Krauskopf, Lewis (August 6, 2007). "Pfizer wins U.S. approval for new HIV drug". Reuters. Archived from the original on 2008-01-04. Retrieved 2007-08-06.
- ↑ World Health Organization (2005). "International nonproprietary names for pharmaceutical substances (INN): recommended INN: list 53". WHO Drug Information. 19 (1): 84–5. hdl:10665/73323. License: CC BY-NC-SA 3.0 IGO.
- ↑ Reshef R, Luger SM, Hexner EO, Loren AW, Frey NV, Nasta SD, et al. (July 2012). "Blockade of lymphocyte chemotaxis in visceral graft-versus-host disease". N. Engl. J. Med. 367 (2): 135–45. doi:10.1056/NEJMoa1201248. PMC 3568501. PMID 22784116.
- ↑ "HIV Drug Reduces Graft-versus-Host Disease in Bone Marrow Transplant Patients, Penn Study Shows". Penn Medicine (Press release). Archived from the original on 2021-05-21. Retrieved 2021-07-02.
External links
- Dean L (2015). "Maraviroc Therapy and CCR5 Genotype". In Pratt VM, McLeod HL, Rubinstein WS, et al. (eds.). Medical Genetics Summaries. National Center for Biotechnology Information (NCBI). PMID 28520358. Bookshelf ID: NBK279895. Archived from the original on 2020-10-26. Retrieved 2021-07-02.
- "Maraviroc". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 2016-08-19. Retrieved 2021-07-02.
- Maraviroc at the US National Library of Medicine Medical Subject Headings (MeSH)
| Identifiers: |
|
|---|
- Pages using duplicate arguments in template calls
- Drugs with non-standard legal status
- Articles with changed InChI identifier
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without UNII source
- Drugs missing an ATC code
- Drug has EMA link
- Drugboxes which contain changes to verified fields
- Drugboxes which contain changes to watched fields
- Articles with changed ChemSpider identifier
- Articles with changed EBI identifier
- Portal templates with all redlinked portals
- Entry inhibitors
- Pfizer brands
- Carboxamides
- Hepatotoxins
- Organofluorides
- Triazoles
- Phenyl compounds
- Tropanes
- Isopropyl compounds
- Cyclohexanes
- RTT