Atazanavir
 | |
 | |
| Names | |
|---|---|
| Pronunciation | /ˌætəˈzænəvɪər/ AT-ə-ZAN-ə-veer[1] |
| Trade names | Reyataz, Evotaz, others[2] |
| |
| Clinical data | |
| Drug class | Protease inhibitor (PI)[2] |
| Main uses | HIV/AIDS[3] |
| Side effects | Headache, nausea, yellowish skin, abdominal pain, trouble sleeping, fever[2] |
| Pregnancy category |
|
| Routes of use | By mouth |
| Defined daily dose | 300 mg [4] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a603019 |
| Legal | |
| License data | |
| Legal status | |
| Pharmacokinetics | |
| Bioavailability | 60-68% |
| Protein binding | 86% |
| Metabolism | Liver (CYP3A4-mediated) |
| Elimination half-life | 6.5 hours |
| Excretion | Fecal and kidney |
| Chemical and physical data | |
| Formula | C38H52N6O7 |
| Molar mass | 704.869 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Atazanavir, sold under the trade name Reyataz among others, is an antiretroviral medication used to treat and prevent HIV/AIDS.[2] It is generally recommended for use with other antiretrovirals.[2] It may be used for prevention after a needlestick injury or other potential exposure.[2] It is taken by mouth once a day.[2]
Common side effects include headache, nausea, yellowish skin, abdominal pain, trouble sleeping, and fever.[2] Severe side effects include rashes such as erythema multiforme and high blood sugar.[2] Atazanavir appears to be safe to use during pregnancy.[2] It is of the protease inhibitor (PI) class and works by blocking HIV protease.[2]
Atazanavir was approved for medical use in the United States in 2003.[2] It was on the World Health Organization's List of Essential Medicines by itself, but is now only on the list as atazanavir/ritonavir.[5][6] In the United States it is not available as a generic medication.[7] The wholesale cost in the developing world is about US$15.72 per month.[8] As of 2015, the cost for a typical month of medication in the United States was more than $200.[7]
Medical uses
Atazanavir is used in the treatment of HIV. The efficacy of atazanavir has been assessed in a number of well designed trials in ART-naive and ART-experienced adults.[9]
Atazanavir is distinguished from other PIs in that it has lesser effects on lipid profile and appears to be less likely to cause lipodystrophy. There may be some cross-resistant with other PIs.[2] When boosted with ritonavir it is equivalent in potency to lopinavir for use in salvage therapy in people with a degree of drug resistance, although boosting with ritonavir reduces the metabolic advantages of atazanavir.[citation needed]
Pregnancy
Atazanavir is pregnancy category B in the United States, meaning that no evidence of harm has been found among pregnant women taking this medication. It is one of the preferred HIV medication to use in pregnant women who have not taken an HIV medication before.[10] It was not associated with any birth defects among over 2,500 live births observed. Atazanavir resulted in a better cholesterol profile and confirmed that it is a safe option during pregnancy.[10]
Dosage
The defined daily dose is 300 mg (by mouth).[4] The typical dose for those 20 to 25 kg is 200 mg once per day while the typical dose in those more than 25 kg is 300 mg once per day.[3]
Contraindications
Atazanavir is contraindicated in those with previous hypersensitivity (e.g., Stevens-Johnson syndrome, erythema multiforme, or toxic skin eruptions). Additionally, atazanavir should not be given with alfuzosin, rifampin, irinotecan, lurasidone, pimozide, triazolam, orally administered midazolam, ergot derivatives, cisapride, St. John's wort, lovastatin, simvastatin, sildenafil, indinavir, or nevirapine.[11]
Side effects
Common side effects include: nausea, jaundice, rash, headache, abdominal pain, vomiting, insomnia, peripheral neurologic symptoms, dizziness, muscle pain, diarrhea, depression and fever.[11] Bilirubin levels in the blood are normally asymptomatically raised with atazanavir, but can sometimes lead to jaundice.
Mechanism of action
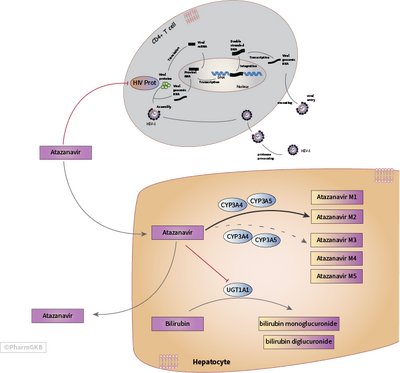
Atazanavir binds to the active site HIV protease and prevents it from cleaving the pro-form of viral proteins into the working machinery of the virus.[13]
If the HIV protease enzyme does not work, the virus is not infectious, and no mature virions are made.[14][15]
The azapeptide drug was designed as an analog of the peptide chain substrate that HIV protease would cleave normally into active viral proteins. More specifically, atazanavir is a structural analog of the transition state during which the bond between a phenylalanine and proline is broken.[16][17]
Humans do not have any enzymes that break bonds between phenylalanine and proline, so this drug will not target human enzymes.
Society and culture

Formulations
Atazanavir is available as a 150 mg capsule, 200 mg capsule, 300 mg capsule, and 50 mg oral powder packet.[11]
The 300 mg capsule should reduce pill burden, as one 300 mg capsule may replace two 150 mg capsules.
References
- ↑ "Atazanavir". MedlinePlus. National Institutes of Health. October 15, 2012. Archived from the original on August 2, 2013. Retrieved August 3, 2013.
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 "Atazanavir Sulfate". The American Society of Health-System Pharmacists. Archived from the original on 20 December 2016. Retrieved 28 November 2016.
- ↑ 3.0 3.1 "ATAZANAVIR = ATV - Essential drugs". medicalguidelines.msf.org. Archived from the original on 27 August 2021. Retrieved 26 August 2020.
- ↑ 4.0 4.1 "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 21 October 2020. Retrieved 21 September 2020.
- ↑ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ↑ "eEML - Electronic Essential Medicines List". list.essentialmeds.org. Archived from the original on 7 December 2022. Retrieved 15 September 2023.
- ↑ 7.0 7.1 Hamilton, Richart (2015). Tarascon Pocket Pharmacopoeia 2015 Deluxe Lab-Coat Edition. Jones & Bartlett Learning. p. 67. ISBN 9781284057560.
- ↑ "Atazanavir". International Drug Price Indicator Guide. Archived from the original on 22 January 2018. Retrieved 28 November 2016.
- ↑ 10.0 10.1 "What's New in the Guidelines? | Adult and Adolescent ARV Guidelines | AIDSinfo". AIDSinfo. Archived from the original on 2016-11-15. Retrieved 2016-11-10.
- ↑ 11.0 11.1 11.2 "Reyataz Package Insert" (PDF). Drugs@FDA. Food and Drug Administration. September 2016. Archived (PDF) from the original on November 11, 2016. Retrieved November 10, 2016.
- ↑ "PharmGKB". PharmGKB. Retrieved 11 January 2024.
- ↑ "Atazanavir". DrugBank. 9 November 2016. Archived from the original on 9 November 2016.
- ↑ Kohl, NE; Emini, EA; Schleif, WA; Davis, LJ; Heimbach, JC; Dixon, RA; Scolnick, EM; Sigal, IS (1 July 1988). "Active human immunodeficiency virus protease is required for viral infectivity". Proceedings of the National Academy of Sciences of the United States of America. 85 (13): 4686–4690. Bibcode:1988PNAS...85.4686K. doi:10.1073/pnas.85.13.4686. ISSN 0027-8424. PMC 280500. PMID 3290901.
- ↑ Lv, Z; Chu, Y; Wang, Y (2015). "HIV protease inhibitors: a review of molecular selectivity and toxicity. ". HIV/AIDS – Research and Palliative Care. 7: 95–104. doi:10.2147/HIV.S79956. PMC 4396582. PMID 25897264.
- ↑ Graziani, Amy L (June 17, 2014). "HIV protease inhibitors". UpToDate.
- ↑ Bold, G; Fässler, A; Capraro, HG; Cozens, R; Klimkait, T; Lazdins, J; Mestan, J; Poncioni, B; Rösel, J; Stover, D; Tintelnot-Blomley, M; Acemoglu, F; Beck, W; Boss, E; Eschbach, M; Hürlimann, T; Masso, E; Roussel, S; Ucci-Stoll, K; Wyss, D; Lang, M (August 1998). "New Aza-Dipeptide Analogues as Potent and Orally Absorbed HIV-1 Protease Inhibitors: Candidates for Clinical Development". Journal of Medicinal Chemistry. 41 (18): 3387–3401. doi:10.1021/jm970873c. PMID 9719591.
External links
| External sites: | |
|---|---|
| Identifiers: |
|
- Pages using duplicate arguments in template calls
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without UNII source
- Drugs missing an ATC code
- Drug has EMA link
- Drugboxes which contain changes to verified fields
- All articles with unsourced statements
- Articles with unsourced statements from December 2016
- Articles with invalid date parameter in template
- Articles with changed KEGG identifier
- Bristol-Myers Squibb
- HIV protease inhibitors
- Pyridines
- RTT