Posaconazole
 | |
 | |
| Names | |
|---|---|
| Trade names | Noxafil, Posanol, others |
| |
| Clinical data | |
| Drug class | Antifungal (azole)[1] |
| Main uses | Thrush, aspergillosis, mucormycosis, coccidioidomycosis, fusariosis, chromoblastomycosis[2][1] |
| Side effects | Nausea, diarrhea, fever, liver problems[2] |
| Pregnancy category | |
| Routes of use | By mouth (oral suspension, delayed-release tablets), IV |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a607036 |
| Legal | |
| License data |
|
| Legal status | |
| Pharmacokinetics | |
| Bioavailability | High |
| Protein binding | 98 to 99% |
| Metabolism | Liver (glucuronidation) |
| Elimination half-life | 16 to 31 hours |
| Excretion | Fecal (71–77%) and kidney (13–14%) |
| Chemical and physical data | |
| Formula | C37H42F2N8O4 |
| Molar mass | 700.778 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Posaconazole, sold under the brand names Noxafil among others, is an antifungal medication use to treat and prevent thrush, aspergillosis, mucormycosis, coccidioidomycosis, fusariosis, and chromoblastomycosis.[2][1] It is used when other treatments are not appropriate.[2] It is taken by mouth of given by injection into a vein.[2]
Common side effects include nausea, diarrhea, fever, and liver problems.[2] Other side effects may include allergic reactions and QT prolongation.[1] Safety is unclear in pregnancy, with use not recommended in early pregnancy.[1] It is in the azole family of medications and works by blocking lanosterol 14 alpha-demethylase.[1]
Posaconazole was approved for medical use in Europe in 2005 and the United States in 2006.[2][1] Generic versions have been approved.[4][5] In the United Kingdom 24 tablets of 100 mg cost the NHS about £600 as of 2021.[5] This amount in the United States costs about 410 USD.[6]
Medical uses
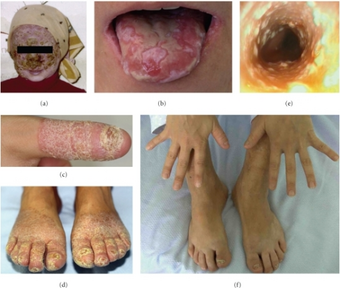
Posaconazole is used to treat invasive Aspergillus and Candida and fungal infections caused by Scedosporium and Fusarium species, which may occur in immunocompromised patients.[7] It is also used for the treatment of oropharyngeal candidiasis (OPC), including OPC refractory to itraconazole and/or fluconazole therapy.[7]
It is also used to treat invasive infections by Candida, Mucor, and Aspergillus species in severely immunocompromised patients.[8][9]
Clinical evidence for its utility in treatment of invasive disease caused by Fusarium species (fusariosis) is limited.[10]
It appears to be helpful in a mouse model of naegleriasis.[11]
Dosage
For thrush it is taken at an initial dose of 200 mg by mouth, than is taken as 100 mg per day for 13 days.[5] Other infections are treated with 400 mg twice per day by mouth or 300 mg twice per day by injection followed by 300 mg once per day by injection.[5]
Pharmacology
Pharmacodynamics
Posaconazole works by disrupting the close packing of acyl chains of phospholipids, impairing the functions of certain membrane-bound enzyme systems such as ATPase and enzymes of the electron transport system, thus inhibiting growth of the fungi. It does this by blocking the synthesis of ergosterol by inhibiting of the enzyme lanosterol 14α-demethylase and accumulation of methylated sterol precursors. Posaconazole is significantly more potent at inhibiting 14-alpha demethylase than itraconazole.[12][13][14]
Microbiology
Posaconazole is active against the following microorganisms:[12][15]
- Candida spp.
- Aspergillus spp.
- Zygomycetes spp.
Pharmacokinetics
Posaconazole is absorbed within three to five hours. It is predominately eliminated through the liver, and has a half-life of about 35 hours. Oral administration of posaconazole taken with a high-fat meal exceeds 90% bioavailability and increases the concentration by four times compared to fasting state.[15][16]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 "Posaconazole Monograph for Professionals". Drugs.com. Archived from the original on 19 October 2021. Retrieved 29 October 2021.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 "Noxafil". Archived from the original on 19 October 2021. Retrieved 29 October 2021.
- ↑ 3.0 3.1 "Posaconazole (Noxafil) Use During Pregnancy". Drugs.com. 23 April 2019. Archived from the original on 30 January 2020. Retrieved 30 January 2020.
- ↑ "Posaconazole: FDA-Approved Drugs". U.S. Food and Drug Administration (FDA). Archived from the original on 20 October 2020. Retrieved 15 August 2020.
- ↑ 5.0 5.1 5.2 5.3 BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 638. ISBN 978-0857114105.
- ↑ "Posaconazole Prices, Coupons & Savings Tips - GoodRx". GoodRx. Retrieved 29 October 2021.
- ↑ 7.0 7.1 "Noxafil- posaconazole suspension Noxafil- posaconazole tablet, coated Noxafil- posaconazole solution". DailyMed. 20 March 2020. Archived from the original on 26 February 2021. Retrieved 15 August 2020.
- ↑ Li X, Brown N, Chau AS, López-Ribot JL, Ruesga MT, Quindos G, et al. (January 2004). "Changes in susceptibility to posaconazole in clinical isolates of Candida albicans". The Journal of Antimicrobial Chemotherapy. 53 (1): 74–80. doi:10.1093/jac/dkh027. PMID 14657086.
- ↑ Walsh TJ, Raad I, Patterson TF, Chandrasekar P, Donowitz GR, Graybill R, et al. (January 2007). "Treatment of invasive aspergillosis with posaconazole in patients who are refractory to or intolerant of conventional therapy: an externally controlled trial". Clinical Infectious Diseases. 44 (1): 2–12. doi:10.1086/508774. JSTOR 4485188. PMID 17143808. – via JSTOR (subscription required)
- ↑ Raad II, Hachem RY, Herbrecht R, Graybill JR, Hare R, Corcoran G, Kontoyiannis DP (May 2006). "Posaconazole as salvage treatment for invasive fusariosis in patients with underlying hematologic malignancy and other conditions". Clinical Infectious Diseases. 42 (10): 1398–403. doi:10.1086/503425. PMID 16619151.
- ↑ Colon BL, Rice CA, Guy RK, Kyle DE (March 2019). "Phenotypic Screens Reveal Posaconazole as a Rapidly Acting Amebicidal Combination Partner for Treatment of Primary Amoebic Meningoencephalitis". The Journal of Infectious Diseases. 219 (7): 1095–1103. doi:10.1093/infdis/jiy622. PMC 6420171. PMID 30358879.
- ↑ 12.0 12.1 Brunton L, Lazo J, Parker K. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 11th ed. San Francisco: McGraw-Hill; 2006. ISBN 978-0-07-142280-2
- ↑ "Clinical Pharmacology Posaconazole". Archived from the original on 30 August 2021. Retrieved 18 February 2010.
- ↑ "Daily Med, Product Information Noxafil". Archived from the original on 21 October 2012. Retrieved 18 February 2010.
- ↑ 15.0 15.1 Ashley ED, Perfect JR (October 2017). "Pharmacology of azoles". In Kauffman CA (ed.). UpToDate. Waltham, MA: UpToDate. Archived from the original on 9 February 2013. Retrieved 18 February 2010.
- ↑ "Drugs at FDA: Noxafil" (PDF). Archived (PDF) from the original on 16 October 2012. Retrieved 18 February 2010.
External links
| External sites: | |
|---|---|
| Identifiers: |
|
- Pages using duplicate arguments in template calls
- Subscription required using via
- Pages containing links to subscription-only content
- Use dmy dates from January 2020
- Articles with invalid date parameter in template
- Drugs with non-standard legal status
- Chem-molar-mass both hardcoded and calculated
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without UNII source
- Drugs missing an ATC code
- Drug has EMA link
- Drugboxes which contain changes to verified fields
- Drugboxes which contain changes to watched fields
- Articles with changed EBI identifier
- 1,2,4-triazol-3-ones
- 27-Hydroxylase inhibitors
- Fluoroarenes
- Lanosterol 14α-demethylase inhibitors
- Merck & Co. brands
- Orphan drugs
- Para-Methoxyphenylpiperazines
- Schering-Plough brands
- Secondary alcohols
- Tetrahydrofurans
- Triazole antifungals
- Ureas
- RTT