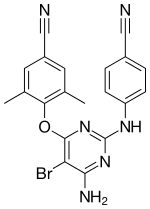
Etravirine
 | |
 | |
| Names | |
|---|---|
| Trade names | Intelence |
| Other names | TMC125 |
| |
| Clinical data | |
| Drug class | NNRTI[1] |
| Main uses | Treat and prevent HIV/AIDS[2] |
| Side effects | Rash, diarrhea, nausea, peripheral nerve problems, headache[1][2] |
| Pregnancy category |
|
| Routes of use | By mouth |
| Typical dose | 200 mg BID[3] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a608016 |
| Legal | |
| License data |
|
| Legal status |
|
| Pharmacokinetics | |
| Protein binding | 99.9% |
| Metabolism | Liver (CYP3A4, CYP2C9 & CYP2C19-mediated) |
| Elimination half-life | 41±20 hours |
| Excretion | Faeces (93.7%), urine (1.2%) |
| Chemical and physical data | |
| Formula | C20H15BrN6O |
| Molar mass | 435.285 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Etravirine (ETR) sold under the brand name Intelence, is a medication used to treat and prevent HIV/AIDS.[2] It is used in people who have been previously treated, together with other antiretroviral medications.[1][4] It is taken by mouth twice per day.[2]
Common side effects include rash, diarrhea, nausea, peripheral nerve problems, and headache.[1][2] Other side effects may include Stevens-Johnson syndrome, immune reconstitution syndrome, and lipodystrophy.[2] Safety in pregnancy is unclear.[2] It is a non-nucleoside reverse transcriptase inhibitor (NNRTI), which blocks reverse transcriptase.[1]
Etravirine was approved for medical use in the Canada, Europe, and the United States in 2008.[2][5][1] It is available as a generic medication.[6] In the United Kingdom a month of treatment costs the NHS about £300 as of 2021.[3] This amount in the United States costs about 380 USD.[6]
Medical uses
Etravirine, in combination with other anti-retrovirals, is indicated for the treatment of human immunodeficiency virus type 1 (HIV-1) infection in antiretroviral treatment-experienced adults, who have evidence of viral replication and HIV-1 strains resistant to a non-nucleoside reverse transcriptase inhibitor (NNRTI) and other antiretroviral agents.
Unlike some agents in the class, resistance to other NNRTIs does not seem to confer resistance to etravirine.[7]
Dosage
The recommended dose of etravirine is 200 mg (2 x 100 mg tablets, or 1 x 200 mg tablet) taken twice daily following a meal. The type of food does not affect the exposure to etravirine.[8]
Side effects
In 2009, the prescribing information for etravirine was modified to include "postmarketing reports of cases of Stevens–Johnson syndrome, toxic epidermal necrolysis and erythema multiforme, as well as hypersensitivity reactions characterized by rash, constitutional findings, and sometimes organ dysfunction, including liver failure. Intelence therapy should be immediately discontinued when signs and symptoms of severe skin or hypersensitivity reactions develop."[9]
Each 100 mg etravirine tablet contains 160 mg of lactose. Patients with rare hereditary problems of galactose intolerance, the Lapp lactase deficiency or glucose-galactose malabsorption should not take this medicine.[10]
Mechanism of action
Etravirine is a second-generation non-nucleoside reverse transcriptase inhibitor (NNRTI), designed to be active against HIV with mutations that confer resistance to the two most commonly prescribed first-generation NNRTIs, mutation K103N for efavirenz and Y181C for nevirapine.[11] This potency appears to be related to etravirine's flexibility as a molecule. Etravirine is a diarylpyrimidine (DAPY), a type of organic molecule with some conformational isomerism that can bind the enzyme reverse transcriptase in multiple conformations, allowing for a more robust interaction between etravirine and the enzyme, even in the presence of mutations.[12] Other diarylpyrimidine-analogues are currently being used as anti-HIV agents, notably rilpivirine.
Society and culture
It is also available in Israel, Russia, Australia, and the European Union,[13] and is under regulatory review in Switzerland.[14]
Research
In a paper etravirine was shown to cause an increase in frataxin production. Frataxin deficiency is a key component to Friedreich's ataxia, a genetically inherited disease that causes the progressive loss of coordination and muscle strength leading to motor incapacitation and the full-time use of a wheelchair.[15]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 "Intelence". Archived from the original on 18 November 2021. Retrieved 16 December 2021.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 "Etravirine Monograph for Professionals". Drugs.com. Archived from the original on 31 July 2020. Retrieved 16 December 2021.
- ↑ 3.0 3.1 BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 684. ISBN 978-0857114105.
- ↑ "Etravirine" (PDF). March 2009. Archived (PDF) from the original on 15 April 2021. Retrieved 16 December 2021.
- ↑ "Newsletter July 2008". pmprb-cepmb.gc.ca. 20 June 2014. Archived from the original on 18 October 2020. Retrieved 16 December 2021.
- ↑ 6.0 6.1 "Etravirine Prices, Coupons & Savings Tips - GoodRx". GoodRx. Archived from the original on 5 November 2016. Retrieved 16 December 2021.
- ↑ Stellbrink HJ (October 2007). "Antiviral drugs in the treatment of AIDS: what is in the pipeline?". Eur. J. Med. Res. 12 (9): 483–95. PMID 17933730.
- ↑ "Intelence prescribing information" (PDF). FDA. Archived (PDF) from the original on July 29, 2018. Retrieved January 19, 2012.
- ↑ "FDA Medwatch Safety Information". Archived from the original on 2017-01-18. Retrieved 2009-08-27.
- ↑ "Etravine: Summary of product characteristics" (PDF). EMEA. p. 5. Archived (PDF) from the original on August 20, 2016. Retrieved July 13, 2011.
- ↑ Evans, David (2008-01-15). "Etravirine—Countdown to Launch". AIDSmeds.com. Archived from the original on 2008-01-19. Retrieved 2008-02-02.
- ↑ Das K, Clark AD, Lewi PJ, Heeres J, De Jonge MR, Koymans LM, Vinkers HM, Daeyaert F, Ludovici DW, Kukla MJ, De Corte B, Kavash RW, Ho CY, Ye H, Lichtenstein MA, Andries K, Pauwels R, De Béthune MP, Boyer PL, Clark P, Hughes SH, Janssen PA, Arnold E (May 2004). "Roles of conformational and positional adaptability in structure-based design of TMC125-R165335 (etravirine) and related non-nucleoside reverse transcriptase inhibitors that are highly potent and effective against wild-type and drug-resistant HIV-1 variants". J. Med. Chem. 47 (10): 2550–60. doi:10.1021/jm030558s. PMID 15115397.
- ↑ "Intelence receives marketing authorisation in the European Union for HIV combination therapy". Tibotec. Archived from the original on 2011-09-28. Retrieved 2008-08-29.
- ↑ "Etravirine (TMC125, Intelence) granted accelerated approval in US". aidsmap. Archived from the original on 2010-01-02. Retrieved 2008-01-24.
- ↑ Alfedi, Giulia; Luffarelli, Riccardo; Condò, Ivano; Pedini, Giorgia; Mannucci, Liliana; Massaro, Damiano S.; Benini, Monica; Toschi, Nicola; Alaimo, Giorgia; Panarello, Luca; Pacini, Laura; Fortuni, Silvia; Serio, Dario; Malisan, Florence; Testi, Roberto; Rufini, Alessandra (2019). "Drug repositioning screening identifies etravirine as a potential therapeutic for friedreich's ataxia". Movement Disorders. 34 (3): 323–334. doi:10.1002/mds.27604. PMID 30624801. S2CID 58567610.
External links
| External sites: | |
|---|---|
| Identifiers: |
|
- Pages using duplicate arguments in template calls
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without UNII source
- Drugs missing an ATC code
- Drug has EMA link
- Drugboxes which contain changes to verified fields
- Drugboxes which contain changes to watched fields
- Articles with changed DrugBank identifier
- Portal templates with all redlinked portals
- Hepatotoxins
- Non-nucleoside reverse transcriptase inhibitors
- Aminopyrimidines
- Johnson & Johnson brands
- Nitriles
- Organobromides
- Belgian inventions
- RTT