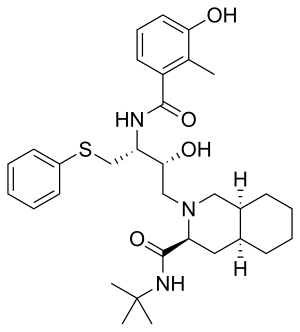
Nelfinavir
 | |
 | |
| Names | |
|---|---|
| Trade names | Viracept |
| |
| Clinical data | |
| Drug class | HIV protease inhibitor[1] |
| Main uses | HIV/AIDS[1] |
| Side effects | Diarrhea, nausea, rash[1] |
| Routes of use | By mouth |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a697034 |
| Legal | |
| License data |
|
| Legal status |
|
| Pharmacokinetics | |
| Bioavailability | Uncertain; increases when taking with food[2] |
| Protein binding | >98% |
| Metabolism | Liver by CYP including CYP3A4 and CYP2C19 |
| Elimination half-life | 3.5–5 hours |
| Excretion | feces (87%), urine (1–2%) |
| Chemical and physical data | |
| Formula | C32H45N3O4S |
| Molar mass | 567.79 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 349.94 °C (661.89 °F) |
| |
| |
Nelfinavir (NFV), sold under the brand name Viracept,is a medication used to treat HIV/AIDS.[1] It is used together with other medications.[1] It is not a first line treatment.[1] It is taken by mouth.[1]
Common side effects include diarrhea, nausea, and rash.[1] Other side effects may include high blood sugar and changes in body fat.[1] While there is no specific harm with us in pregnancy, better options are available.[3] It is an HIV protease inhibitor.[1]
Nelfinavir was patented in 1992 and approved for medical use in 1997.[4] While it was approved in Europe in 1998, this was later withdrawn.[5] In the United States it costs about 1,200 USD per month as of 2021.[6]
Medical uses
Dosage
It is generally take at a dose of 1250 mg twice or 750 mg three times daily.[7]
Side effects
Common (>1%) side effects include insulin resistance, hyperglycemia and lipodystrophy.[8]
Nelfinavir can produce a range of adverse side effects. Flatulence, diarrhea or abdominal pain are common (i.e. experienced by more than one in one hundred patients). Fatigue, urination, rash, mouth ulcers or hepatitis are less frequent effects (experienced by one in one thousand to one in one hundred patients). Nephrolithiasis, arthralgia, leukopenia, pancreatitis or allergic reactions may occur, but are rare (less than one in one thousand patients) .
Interactions
Nelfinavir's interaction profile is similar to that of other protease inhibitors. Most interactions occur at the level of the Cytochrome P450 isozymes 3A4 and CYP2C19, by which nelfinavir is metabolised.
Pharmacology
Nelfinavir should be taken with food. Taking the drug with food decreases the risk of diarrhea as a side effect.
Mechanism of action
Nelfinavir is a protease inhibitor: it inhibits HIV-1 and HIV-2 proteases. HIV protease is an aspartate protease which splits viral protein molecules into smaller fragments, and it is vital to both the replication of the virus within the cell, and also to the release of mature viral particles from an infected cell. Nelfinavir is a competitive inhibitor[8] (2 nM) which is designed to bind tightly and is not cleaved due to the presence of a hydroxyl group as opposed to a keto group in the middle amino acid residue mimic, which would be otherwise S-phenylcysteine. All protease inhibitors bind to the protease, the precise mode of binding determines how the molecule inhibits the protease. The way Nelfinavir binds the enzyme may be sufficiently unique to reduce cross-resistance[clarification needed] between it and other PIs. Also, not all PIs inhibit both HIV-1 and HIV-2 proteases.
History
Nelfinavir was developed by Agouron Pharmaceuticals as part of a joint venture with Eli Lilly and Company.[9] Agouron Pharmaceuticals was acquired by Warner Lambert in 1999 and is now a subsidiary of Pfizer. It is marketed in Europe by Hoffman-La Roche and elsewhere by ViiV Healthcare.
The U.S. Food and Drug Administration (FDA) approved it for therapeutic use on March 14, 1997,[citation needed] making it the twelfth[citation needed] approved antiretroviral. The initial product launched proved to be the largest[citation needed] "biotech launch" in the history of the pharmaceutical industry, achieving first full year sales exceeding $US335M.[citation needed] Agouron's patent on the drug expired in 2014.
On the 6 June 2007, both the Medicines and Healthcare products Regulatory Agency and the European Medicines Agency[10] put out an alert requesting the recall of any of the drug in circulation, because some batches may have been contaminated with potentially cancer-causing chemicals.
Synthesis

Research
Antiviral
Nelfinavir inhibits maturation and export of the herpes simplex 1 virus[12] and the Kaposi's sarcoma virus.[13] Nelfinavir is being tested to treat COVID-19.
Cancer
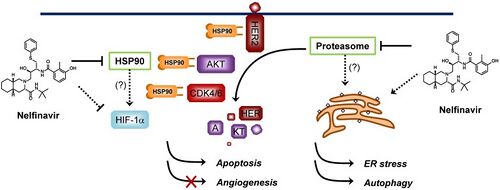
Since 2009, nelfinavir has been under investigation for potential use as an anti-cancer agent.[14] When applied to cancer cells in culture (in vitro), it can inhibit the growth of a variety of cancer types and can trigger cell death (apoptosis).[15] When Nelfinavir was given to laboratory mice with tumors of the prostate or of the brain, it could suppress tumor growth in these animals.[16][17] At the cellular level, nelfinavir exerts multiple effects to inhibit cancer growth; the two main ones appear to be inhibition of the Akt/PKB signaling pathway and activation of endoplasmic reticulum stress with subsequent unfolded protein response.[18]
In the United States, about three dozen clinical trials are being conducted (or have been completed) in order to determine whether nelfinavir is effective as a cancer therapeutic agent in humans.[19] In some of these trials, nelfinavir is used alone in monotherapy fashion, whereas in others it is combined with other modes of cancer therapy, such as well-established chemotherapeutic agents or radiation therapy.
As of April 2016[update], no phase 3 cancer trials are registered.[20]
Anti-virulence activity
Nelfinavir and simple derivatives have been found to inhibit the production of the virulence factor streptolysin S, a cytolysin produced by the human pathogen Streptococcus pyogenes.[21] Nelfinavir and these related molecules did not exhibit detectable antibiotic activity, but did also inhibit the production of other biologically active molecules, including plantazolicin (antibiotic), listeriolysin S (cytolysin), and clostridiolysin S (cytolysin), by other bacteria.[21]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 1.9 "Nelfinavir Monograph for Professionals". Drugs.com. Archived from the original on 23 January 2021. Retrieved 12 November 2021.
- ↑ "Viracept (nelfinavir mesylate) Tablets and Oral Powder, for Oral Use. Full Prescribing Information" (PDF). ViiV Healthcare Company. Research Triangle Park, NC 27709. Archived (PDF) from the original on 4 March 2016. Retrieved 23 November 2015.
- ↑ "Nelfinavir (Viracept) Use During Pregnancy". Drugs.com. Archived from the original on 23 January 2021. Retrieved 12 November 2021.
- ↑ Fischer, Jnos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 510. ISBN 9783527607495. Archived from the original on 2021-05-24. Retrieved 2021-04-24.
- ↑ "Viracept". Archived from the original on 23 October 2020. Retrieved 12 November 2021.
- ↑ "Nelfinavir Prices, Coupons & Savings Tips - GoodRx". GoodRx. Retrieved 12 November 2021.
- ↑ "Nelfinavir". LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. National Institute of Diabetes and Digestive and Kidney Diseases. 2012. Archived from the original on 2021-05-06. Retrieved 2021-11-12.
- ↑ 8.0 8.1 8.2 Shim, JS; Liu, JO (2014). "Recent Advances in Drug Repositioning for the Discovery of New Anticancer Drugs". Int. J. Biol. Sci. 10 (7): 654–63. doi:10.7150/ijbs.9224. PMC 4081601. PMID 25013375.
- ↑ Kaldor SW, Kalish VJ, et al. (1997). "Viracept (nelfinavir mesylate, AG1343): A potent, orally bioavailable inhibitor of HIV-1 protease". Journal of Medicinal Chemistry. 40 (24): 3979–3985. doi:10.1021/jm9704098. PMID 9397180.
- ↑ Press release Archived 2007-06-10 at the Wayback Machine from the European Medicines Agency regarding possible genotoxic ethyl mesylate contamination
- ↑ Drugs of the Future, Volume 22, Issue 4, April 1997; Nelfinavir Mesylate. Antiviral for AIDS, HIV-1 protease inhibitor. X. Rabasseda, A.M. Martel, J. Castañer.
- ↑ Ambinder, Richard F.; Dennis, Phillip A.; Gibson, Wade; Shirley, Courtney M.; Desai, Prashant J.; Kalu, Nene N. (15 May 2014). "Nelfinavir Inhibits Maturation and Export of Herpes Simplex Virus 1". Journal of Virology. 88 (10): 5455–5461. doi:10.1128/JVI.03790-13. PMC 4019105. PMID 24574416 – via jvi.asm.org.
- ↑ Vieira, Jeffrey; Lagunoff, Michael; Casper, Corey; Corey, Lawrence; Geballe, Adam P.; Gray, Matthew; Gachelet, Eliora; Ikoma, Minako; Carlsson, Jacquelyn; Gantt, Soren (1 June 2011). "The HIV Protease Inhibitor Nelfinavir Inhibits Kaposi's Sarcoma-Associated Herpesvirus Replication In Vitro". Antimicrobial Agents and Chemotherapy. 55 (6): 2696–2703. doi:10.1128/AAC.01295-10. PMC 3101462. PMID 21402841 – via aac.asm.org.
- ↑ Chow WA, Jiang C, Guan M (2009). "Anti-HIV drugs for cancer therapeutics: back to the future?". Lancet Oncol. 10 (1): 61–71. doi:10.1016/S1470-2045(08)70334-6. PMID 19111246.
- ↑ Gills, JJ; Lopiccolo, J; Tsurutani, J; Shoemaker, RH; Best, CJM; Abu-Asab, MS; Borojerdi, J; Warfel, NA; et al. (September 2007). "Nelfinavir, A lead HIV protease inhibitor, is a broad-spectrum, anticancer agent that induces endoplasmic reticulum stress, autophagy, and apoptosis in vitro and in vivo". Clinical Cancer Research. 13 (17): 5183–94. doi:10.1158/1078-0432.CCR-07-0161. PMID 17785575. Archived from the original on 2016-08-08. Retrieved 2021-04-24.
- ↑ Pyrko P, Kardosh A, Wang W, Xiong W, Schönthal AH, Chen TC (2007). "HIV-1 protease inhibitors nelfinavir and atazanavir induce malignant glioma death by triggering endoplasmic reticulum stress". Cancer Research. 67 (22): 10920–8. doi:10.1158/0008-5472.CAN-07-0796. PMID 18006837. Archived from the original on 2016-08-08. Retrieved 2021-04-24.
- ↑ Yang, Y; Ikezoe, T; Takeuchi, T; Adachi, Y; Ohtsuki, Y; Takeuchi, S; Koeffler, HP; Taguchi, H (July 2005). "HIV-1 protease inhibitor induces growth arrest and apoptosis of human prostate cancer LNCaP cells in vitro and in vivo in conjunction with blockade of androgen receptor STAT3 and AKT signaling". Cancer Science. 96 (7): 425–33. doi:10.1111/j.1349-7006.2005.00063.x. PMID 16053514. S2CID 44702882.
- ↑ Koltai T. (2015). "Nelfinavir and other protease inhibitors in cancer: mechanisms involved in anticancer activity". F1000Res. 4 (2): 9. doi:10.12688/f1000research.5827.2. PMC 4457118. PMID 26097685. Archived from the original on 2021-08-10. Retrieved 2021-04-24.
- ↑ "Search of: Nelfinavir cancer - List Results - ClinicalTrials.gov". clinicaltrials.gov. Archived from the original on 2020-04-04. Retrieved 2021-04-24.
- ↑ "Search of: Nelfinavir - Phase 3 - List Results - ClinicalTrials.gov". clinicaltrials.gov. Archived from the original on 2020-04-04. Retrieved 2021-04-24.
- ↑ 21.0 21.1 Maxson T, Deane CD, Molloy EM, Cox CL, Markley AL, Lee SW, Mitchell DA (2015). "HIV protease inhibitors block streptolysin S production". ACS Chemical Biology. 10 (5): 1217–26. doi:10.1021/cb500843r. PMC 4574628. PMID 25668590.
External links
- Pai, VB; Nahata, MC (March 1999). "Nelfinavir mesylate: a protease inhibitor". The Annals of Pharmacotherapy. 33 (3): 325–39. doi:10.1345/aph.18089. PMID 10200859. S2CID 24066955.
- Bardsley-Elliot, A; Plosker, GL (March 2000). "Nelfinavir: an update on its use in HIV infection". Drugs. 59 (3): 581–620. doi:10.2165/00003495-200059030-00014. PMID 10776836.
| External sites: | |
|---|---|
| Identifiers: |
|
- Pages using duplicate arguments in template calls
- Webarchive template wayback links
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without UNII source
- Drugs missing an ATC code
- Drugboxes which contain changes to verified fields
- Wikipedia articles needing clarification from May 2012
- Articles with invalid date parameter in template
- All articles with unsourced statements
- Articles with unsourced statements from April 2011
- Articles containing potentially dated statements from April 2016
- All articles containing potentially dated statements
- Articles with changed EBI identifier
- Benzamides
- Decahydroisoquinolines
- Hepatotoxins
- HIV protease inhibitors
- Hoffmann-La Roche brands
- Pfizer brands
- Phenols
- Thioethers
- Tert-butyl compounds
- RTT