Cabozantinib
 | |
| Names | |
|---|---|
| Pronunciation | ka" boe zan' ti nib[1] |
| Trade names | Cometriq, Cabometyx, others |
| Other names | XL184, BMS907351 |
| |
| Clinical data | |
| Drug class | Tyrosine kinase inhibitor[2] |
| Main uses | Medullary thyroid cancer, renal cell carcinoma, hepatocellular carcinoma[3][2] |
| Side effects | Diarrhea, tiredness, high blood pressure, nausea, weight loss[2] |
| Pregnancy category |
|
| Routes of use | By mouth |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a613015 |
| Legal | |
| License data |
|
| Legal status | |
| Pharmacokinetics | |
| Protein binding | ≥99.7% |
| Metabolism | Liver (CYP3A4-mediated) |
| Elimination half-life | 110 hours |
| Excretion | Feces (54%), urine (27%) |
| Chemical and physical data | |
| Formula | C28H24FN3O5 |
| Molar mass | 501.514 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Cabozantinib, sold under the brand names Cometriq and Cabometyx among others, is a medication used to treat medullary thyroid cancer, renal cell carcinoma, and hepatocellular carcinoma.[3][2] It is used in advanced disease or cases which have failed other treatments.[2] It is taken by mouth.[2]
Common side effects include diarrhea, tiredness, high blood pressure, nausea, and weight loss.[2] Other side effects may include bleeding, fistula, blood clots, liver problems, and reversible posterior leukoencephalopathy syndrome.[2] Use in pregnancy may harm the baby.[2] It is a tyrosine kinase inhibitor of MET, VEGFR, and AXL.[2]
Cabozantinib was approved for medical use in the United States in 2012 and Europe in 2014.[2][3] In the United Kingdom a dose of 60 mg a day for a month costs about £5,150 as of 2021.[10] This amount in the United States costs about 22,600 USD.[11]
Medical uses
Cabozantinib is used in two forms. A capsule form is used since 2012, to treat medullary thyroid cancer[9][7] and a tablet form is used since 2016, as a second line treatment for renal cell carcinoma.[8][6]
In the United States, cabozantinib (Cabometyx) is also indicated for the treatment of people aged twelve years of age and older with locally advanced or metastatic differentiated thyroid cancer (DTC) that has progressed following prior VEGFR-targeted therapy and who are ineligible or refractory to radioactive iodine.[12]
Dosage
It is generally taken at a dose of 60 mg per day.[2] Doses of of 140 mg per day may be used for thyroid cancer.[10]
The brands Cometriq and Cabometyx have different formulations and are not interchangeable.[13]
Contraindications
Cabozantinib has not been tested in pregnant women; it causes harm to fetuses in rodents. Pregnant women should not take this drug, and women should not become pregnant while taking it. It is not known if cabozantinib is excreted in breast milk.[7][6]
The drug should be used with caution in people with a history of heart rhythm problems, including long QT interval.[6]
Side effects
In the US, the capsule formulation (Cometriq) carries a black box warning of the risk of holes forming in the stomach or intestines as well as formation of fistulas (tunnels between the GI tract and the skin).[9] The black box also warns against the risk of uncontrolled bleeding.[9] The tablet formulation (Cabometyx) warns of these effects as well.[8][6]
The labels also warn of the risk of clots forming and causing heart attacks or strokes, high blood pressure including hypertensive crisis, osteonecrosis of the jaw, severe diarrhea, skin sloughing off the palms and soles, a syndrome with headaches, confusion, loss of vision, and seizures, and protein appearing in urine.[9][8][7][6]
Very common adverse effects (greater than 10% of people) include decreased appetite; low calcium, potassium, phosphate, and magnesium levels; high bilirubin levels; distorted sense of taste, headache, and dizziness; high blood pressure; distorted sense of hearing, earaches and sore throat; diarrhea, nausea, constipation, vomiting, stomach pain and upset stomach, and inflammation of the mouth and lips and a burning sensation in the mouth; skin sloughing off the palms and soles, hair color changes and hair loss, rash, dry skin, and red skin; joint pain and muscle spasms; fatigue and weakness; weight loss, elevated transaminases, higher cholesterol levels, and loss of red and white blood cells.[6]
Common adverse effects (between 1% and 10% of people) include abscesses (inside the body, on the skin, and in teeth skin), pneumonia, inflamed hair follicles, fungal infections, low thyroid levels, dehydration, loss of albumin, anxiety, depression, and confusion, peripheral neuropathy, tingling, and tremor, tinnitus, atrial fibrillation, low blood pressure, blocked veins, paleness, chills, fistulas forming in the trachea and esophagus, blood clots in the lungs, and bleeding in the respiratory tract, GI perforation, bleeding in the stomach and intestines, pancreatitis, hemorrhoids, anal fissure, anal inflammation, gallstones, hard skin growths, acne, blisters, abnormal hair growth, loss of skin color and skin flaking, chest pain, blood or protein in urine, wounds that don't heal well, and facial swelling.[6]
Interactions
Grapefruit and grapefruit juice should be avoided as they may increase the concentration of the drug in the blood.[6]
Cabozantinib is a substrate of CYP3A4 and multidrug resistance-associated protein 2; drugs that inhibit these enzymes will increase the half-life of cabozantinib and potentially increase its adverse effects; drugs that activate them may cause cabozantinib to be less effective.[6]
It inhibits P-glycoprotein, so will change the availability of other drugs that depend on this transporter.[6]
Pharmacology
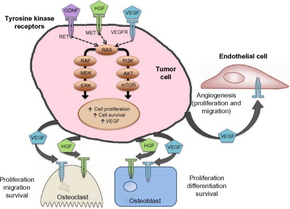
It inhibits the following receptor tyrosine kinases: MET (hepatocyte growth factor receptor protein) and VEGFR, RET, GAS6 receptor (AXL), KIT), and Fms-like tyrosine kinase-3 (FLT3).[6]
History
Cabozantinib was granted orphan drug status by the U.S. Food and Drug Administration (FDA) in November 2010,[14] and in February 2017.[15]
Exelixis filed a new drug application with the FDA in the first half of 2012,[16] and on November 29, 2012 cabozantinib in its capsule formulation was granted marketing approval by the U.S. FDA under the name Cometriq for treating patients with medullary thyroid cancer.[17][18] The capsule form was approved in the European Union for the same purpose in 2014.[3]
In March 2016 Exelixis licensed to Ipsen worldwide rights (outside the US, Canada, and Japan) to market cabozantinib.[19]
Exelixis' Phase III trial results of testing the drug in renal cancer published in the NEJM in 2015.[20] In April 2016 the FDA granted approval for marketing the tablet formulation as a second line treatment for kidney cancer[21][22] and the same was approved in the European Union in September of that year.[5]
In December 2017, the FDA granted approval to cabozantinib (Cabometyx, Exelixis, Inc.) for treatment of people with advanced renal cell carcinoma (RCC).[13] The approval was based on data from CABOSUN (NCT01835158), a randomized, open-label phase II multicenter study in 157 participants with intermediate and poor-risk previously untreated RCC.[13]
In January 2019, the FDA approved cabozantinib (Cabometyx, Exelixis, Inc.) for people with hepatocellular carcinoma (HCC) who have been previously treated with sorafenib.[23] The approval was based on CELESTIAL (NCT01908426), a randomized (2:1), double-blind, placebo-controlled, multicenter trial in participants with HCC who had previously received sorafenib and had Child Pugh Class A liver impairment.
Research
Cabozantinib is being researched[24] for efficacy as a treatment for renal cell carcinoma (RCC), hepatocellular carcinoma (HCC), cervical cancer, colorectal cancer (CRC), urothelial cancer, prostate cancer, gastric and gastroesophageal cancer, bladder cancer, melanoma, merkel cell carcinoma, brain cancers (including glioblastoma multiforme and anaplastic astrocytoma), non-small cell lung cancer (NSCLC), adrenocortical carcinoma, various sarcomas, head and neck squamous cell carcinomas (HNSCC), breast cancer, endometrial cancer, neuroendocrine cancers, and neurofibromatosis type 1.[25]
References
- ↑ "Cabozantinib". LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. National Institute of Diabetes and Digestive and Kidney Diseases. 2012. Archived from the original on 11 January 2022. Retrieved 29 December 2021.
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 "DailyMed - CABOMETYX- cabozantinib tablet". dailymed.nlm.nih.gov. Archived from the original on 29 October 2020. Retrieved 29 December 2021.
- ↑ 3.0 3.1 3.2 3.3 3.4 "Cometriq EPAR". European Medicines Agency (EMA). Archived from the original on 2 October 2018. Retrieved 23 September 2020.
- ↑ "Cabozantinib Use During Pregnancy". Drugs.com. 30 March 2020. Archived from the original on 3 December 2020. Retrieved 23 September 2020.
- ↑ 5.0 5.1 "Cabometyx EPAR". European Medicines Agency (EMA). Archived from the original on 4 August 2020. Retrieved 23 September 2020.
- ↑ 6.00 6.01 6.02 6.03 6.04 6.05 6.06 6.07 6.08 6.09 6.10 6.11 "Cabozantinib tablet (Cabometyx) UK Summary of Product Characteristics". UK Electronic Medicines Compendium. September 2016. Archived from the original on 2019-06-23. Retrieved 2021-10-05.
- ↑ 7.0 7.1 7.2 7.3 "Cabozantinib capsule (Cometriq) UK Summary of Product Characteristics (SPC) - (eMC)". UK Electronic Medicines Compendium. November 2016. Archived from the original on 2019-06-23. Retrieved 2021-10-05.
- ↑ 8.0 8.1 8.2 8.3 "Cabometyx- cabozantinib tablet". DailyMed. 21 July 2020. Archived from the original on 29 October 2020. Retrieved 23 September 2020.
- ↑ 9.0 9.1 9.2 9.3 9.4 "Cometriq- cabozantinib kit Cometriq- cabozantinib capsule". DailyMed. 11 February 2020. Archived from the original on 29 October 2020. Retrieved 23 September 2020.
- ↑ 10.0 10.1 BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 1017. ISBN 978-0857114105.
- ↑ "Cabometyx Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 4 March 2021. Retrieved 29 December 2021.
- ↑ "FDA approves cabozantinib for differentiated thyroid cancer". U.S. Food and Drug Administration. 22 September 2021. Archived from the original on 23 September 2021. Retrieved 22 September 2021.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ 13.0 13.1 13.2 "FDA grants regular approval to Cabometyx for first-line treatment of a". U.S. Food and Drug Administration (FDA). 19 December 2017. Archived from the original on 12 June 2019. Retrieved 23 September 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "Cabozantinib Orphan Drug Designations and Approvals". U.S. Food and Drug Administration (FDA). 29 November 2010. Archived from the original on 12 November 2020. Retrieved 11 November 2020.
- ↑ "Search Orphan Drug Designations and Approvals". U.S. Food and Drug Administration (FDA). 2 March 2017. Archived from the original on 12 November 2020. Retrieved 11 November 2020.
- ↑ "Thyroid cancer drug cabozantinib prolongs PFS". Archived from the original on 2012-04-02. Retrieved 24 October 2011.
- ↑ "FDA approves Cometriq to treat rare type of thyroid cancer" (Press release). U.S. Food and Drug Administration (FDA). 29 November 2012. Archived from the original on July 7, 2014.
- ↑ "Drug Approval Package: Cometriq (cabozantinib) Capsules NDA #203756". U.S. Food and Drug Administration (FDA). Archived from the original on 22 February 2017. Retrieved 23 September 2020.
- Lay summary in: (PDF) https://www.accessdata.fda.gov/drugsatfda_docs/nda/2012/203756Orig1s000SumR.pdf.
{{cite web}}: Missing or empty|title=(help)
- Lay summary in: (PDF) https://www.accessdata.fda.gov/drugsatfda_docs/nda/2012/203756Orig1s000SumR.pdf.
- ↑ Garde D (March 1, 2016). "Ipsen bets up to $855M on Exelixis' once-failed cancer drug". FierceBiotech. Archived from the original on December 2, 2020. Retrieved October 5, 2021.
- ↑ Choueiri TK, Escudier B, Powles T, Mainwaring PN, Rini BI, Donskov F, et al. (November 2015). "Cabozantinib versus Everolimus in Advanced Renal-Cell Carcinoma". The New England Journal of Medicine. 373 (19): 1814–23. doi:10.1056/nejmoa1510016. PMC 5024539. PMID 26406150.
- ↑ "Cabozantinib (Cabometyx)". U.S. Food and Drug Administration (FDA). 25 April 2016. Archived from the original on 23 September 2020. Retrieved 23 September 2020.
- ↑ "Cabometyx (cabozantinib) Tablets". U.S. Food and Drug Administration (FDA). 12 January 2018. Archived from the original on 14 February 2021. Retrieved 23 September 2020.
- ↑ "FDA approves cabozantinib for hepatocellular carcinoma". U.S. Food and Drug Administration (FDA). 14 January 2019. Archived from the original on 23 September 2020. Retrieved 23 September 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "Search of: cabozantinib | Recruiting, Active, not recruiting, Enrolling by invitation Studies - List Results - ClinicalTrials.gov". clinicaltrials.gov. Archived from the original on 2021-08-09. Retrieved 2021-08-09.
- ↑ Fisher, Michael J.; Shih, Chie-Schin; Rhodes, Steven D.; Armstrong, Amy E.; Wolters, Pamela L.; Dombi, Eva; Zhang, Chi; Angus, Steven P.; Johnson, Gary L.; Packer, Roger J.; Allen, Jeffrey C. (13 January 2021). "Cabozantinib for neurofibromatosis type 1–related plexiform neurofibromas: a phase 2 trial". Nature Medicine. 27 (1): 165–173. doi:10.1038/s41591-020-01193-6. ISSN 1546-170X. PMC 8275010. PMID 33442015.
External links
| External sites: | |
|---|---|
| Identifiers: |
|
- "Cabozantinib s-malate". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 2021-02-08. Retrieved 2021-10-05.
- "Cabozantinib s-malate". NCI Drug Dictionary. National Cancer Institute. Archived from the original on 2020-10-20. Retrieved 2021-10-05.
- "Cabozantinib (liver and kidney cancer)". MedlinePlus. Archived from the original on 2021-08-16. Retrieved 2021-10-05.
- Clinical trial number NCT01835158 for "Cabozantinib-s-malate or Sunitinib Malate in Treating Patients With Previously Untreated Locally Advanced or Metastatic Kidney Cancer" at ClinicalTrials.gov
- Clinical trial number NCT01908426 for "Study of Cabozantinib (XL184) vs Placebo in Subjects With Hepatocellular Carcinoma Who Have Received Prior Sorafenib (CELESTIAL)" at ClinicalTrials.gov
- Clinical trial number NCT03690388 for "A Study of Cabozantinib Compared With Placebo in Subjects With Radioiodine-refractory Differentiated Thyroid Cancer Who Have Progressed After Prior Vascular Endothelial Growth Factor Receptor (VEGFR) -Targeted Therapy" at ClinicalTrials.gov
- Pages using duplicate arguments in template calls
- Wikipedia articles incorporating the PD-notice template
- CS1 errors: missing title
- CS1 errors: bare URL
- Drugs with non-standard legal status
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without UNII source
- Chemicals using indexlabels
- Drugs missing an ATC code
- Drug has EMA link
- Articles containing unverified chemical infoboxes
- Antineoplastic drugs
- Breakthrough therapy
- Cyclopropanes
- Fluoroarenes
- Orphan drugs
- Protein kinase inhibitors
- Quinolines
- Tyrosine kinase inhibitors
- RTT