Afatinib
 | |
 | |
| Names | |
|---|---|
| Trade names | Gilotrif, Giotrif, Afanix |
| Other names | BIBW 2992 |
| |
| Clinical data | |
| Drug class | Tyrosine kinase inhibitor[1] |
| Main uses | Non-small cell lung carcinoma (NSCLC)[2] |
| Side effects | Diarrhea, rash, mouth inflammation, dry skin, nausea, itchiness[2] |
| Pregnancy category |
|
| Routes of use | By mouth |
| Typical dose | 40 mg OD[2] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a613044 |
| Legal | |
| License data | |
| Legal status | |
| Pharmacokinetics | |
| Protein binding | 95% |
| Metabolism | CYP not involved |
| Elimination half-life | 37 hours |
| Excretion | Faeces (85%), urine (4%) |
| Chemical and physical data | |
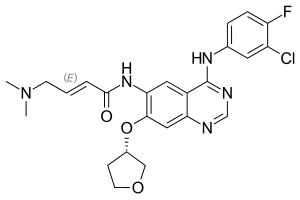
| Formula | C24H25ClFN5O3 |
| Molar mass | 485.94 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Afatinib, sold under the brand name Gilotrif among others, is a medication used to treat non-small cell lung carcinoma (NSCLC).[2] It is mainly used in advanced cases with a non-resistant epidermal growth factor receptor (EGFR) mutation.[2] It is taken by mouth.[2]
Common side effects include diarrhea, rash, mouth inflammation, dry skin, nausea, and itchiness.[2] Other side effects may include interstitial lung disease, liver problems, gastrointestinal perforation, and corneal inflammation.[2] Use in pregnancy may harm the baby.[2] It is a tyrosine kinase inhibitor which blocks a family of proteins known as ErbB.[3][1]
Afatinib was approved for medical use in the United States and Europe in 2013.[2][3] It is on the World Health Organization's List of Essential Medicines as an alternative to erlotinib.[4] In the United Kingdom 4 weeks costs the NHS about £2,000 as of 2021.[5] This amount in the United States costs about 9,800 USD.[6]
Medical uses
It has received regulatory approval for use as a treatment for non-small cell lung cancer,[2][7][8][9] although there is emerging evidence to support its use in other cancers such as breast cancer.[10]
Dosage
It is taken at a dose of 40 mg once per day.[2] This may be increased to 50 mg per day.[5]
Side effects
Side effects by frequency include:[2][7][8][9][11]
- Very common (>10% frequency)
- Diarrhea (>90%)
- Rash/dermatitis acneform
- Stomatitis
- Paronychia
- Decreased appetite
- Nose bleed
- Itchiness
- Dry skin
- Common (1–10% frequency)
- Dehydration
- Taste changes
- Dry eye
- Cystitis
- Cheilitis
- Fever
- Runny/stuffy nose
- Low amount of potassium in the blood
- Conjunctivitis
- Increased ALT
- Increased AST
- Hand-foot syndrome
- Muscle spasms
- Kidney impairment and/or failure
- Uncommon (0.1-1% frequency)
Mechanism of action

Like lapatinib and neratinib, afatinib is a protein kinase inhibitor that also irreversibly inhibits human epidermal growth factor receptor 2 (Her2) and epidermal growth factor receptor (EGFR) kinases. Afatinib is not only active against EGFR mutations targeted by first generation tyrosine-kinase inhibitors (TKIs) like erlotinib or gefitinib, but also against less common mutations which are resistant to these drugs. However, it is not active against the T790M mutation which generally requires third generation drugs like osimertinib.[13] Because of its additional activity against Her2, it is being investigated for breast cancer as well as other EGFR and Her2 driven cancers.[14]
Society and culture
Brand names
In Bangladesh under the trade name Afanix.
References
- ↑ 1.0 1.1 "Afatinib Monograph for Professionals". Drugs.com. Archived from the original on 10 December 2021. Retrieved 13 January 2022.
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 2.13 "Gilotrif (afatinib) tablet, film coated". DailyMed. Boehringer Ingelheim Pharmaceuticals, Inc. 18 October 2019. Archived from the original on 2 February 2014. Retrieved 4 November 2020.
- ↑ 3.0 3.1 "Giotrif". Archived from the original on 22 November 2021. Retrieved 13 January 2022.
- ↑ World Health Organization (2021). World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. hdl:10665/345533. WHO/MHP/HPS/EML/2021.02.
- ↑ 5.0 5.1 BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 1011. ISBN 978-0857114105.
- ↑ "Gilotrif Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 15 January 2021. Retrieved 13 January 2022.
- ↑ 7.0 7.1 "Giotrif Afatinib (as afatinib dimaleate)" (PDF). TGA eBusiness Services. Boehringer Ingelheim Pty Limited. 7 November 2013. Archived from the original on 28 August 2018. Retrieved 28 January 2014.
- ↑ 8.0 8.1 "Giotrif 20 mg film-coated tablets – Summary of Product Characteristics (SPC)". electronic Medicines Compendium. Boehringer Ingelheim Limited. 20 January 2014. Archived from the original on 1 February 2014. Retrieved 28 January 2014.
- ↑ 9.0 9.1 "Giotrif : EPAR -Product Information" (PDF). European Medicines Agency. Boehringer Ingelheim International GmbH. 16 October 2013. Archived (PDF) from the original on 4 February 2014. Retrieved 28 January 2014.
- ↑ Lin NU, Winer EP, Wheatley D, Carey LA, Houston S, Mendelson D, et al. (June 2012). "A phase II study of afatinib (BIBW 2992), an irreversible ErbB family blocker, in patients with HER2-positive metastatic breast cancer progressing after trastuzumab". Breast Cancer Research and Treatment. 133 (3): 1057–65. doi:10.1007/s10549-012-2003-y. PMC 3387495. PMID 22418700.
- ↑ "Gilotrif (afatinib) dosing, indications, interactions, adverse effects, and more". Medscape Reference. WebMD. Archived from the original on 7 March 2021. Retrieved 28 January 2014.
- ↑ Schubert-Zsilavecz, M, Wurglics, M, Neue Arzneimittel Frühjahr 2013. (in German)
- ↑ Li D, Ambrogio L, Shimamura T, Kubo S, Takahashi M, Chirieac LR, et al. (August 2008). "BIBW2992, an irreversible EGFR/HER2 inhibitor highly effective in preclinical lung cancer models". Oncogene. 27 (34): 4702–11. doi:10.1038/onc.2008.109. PMC 2748240. PMID 18408761.
- ↑ Minkovsky N, Berezov A (December 2008). "BIBW-2992, a dual receptor tyrosine kinase inhibitor for the treatment of solid tumors". Current Opinion in Investigational Drugs. 9 (12): 1336–46. PMID 19037840.
External links
| External sites: | |
|---|---|
| Identifiers: |
|
- "Afatinib dimaleate". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 4 November 2021. Retrieved 4 October 2021.
- Pages using duplicate arguments in template calls
- Articles with German-language sources (de)
- Articles with hatnote templates targeting a nonexistent page
- Use dmy dates from February 2015
- Articles with invalid date parameter in template
- Drugs with non-standard legal status
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without UNII source
- Chemicals using indexlabels
- Drugs missing an ATC code
- Drug has EMA link
- Drugboxes which contain changes to verified fields
- Articles with changed KEGG identifier
- Receptor tyrosine kinase inhibitors
- Chloroarenes
- Fluoroarenes
- Quinazolines
- Phenol ethers
- Tetrahydrofurans
- Aromatic amines
- Carboxamides
- Covalent inhibitors
- Dimethylamino compounds
- Enones
- RTT
- World Health Organization essential medicines (alternatives)