Acalabrutinib
 | |
| Names | |
|---|---|
| Trade names | Calquence |
| Other names | ACP-196 |
| |
| Clinical data | |
| Drug class | Bruton's tyrosine kinase inhibitor[1] |
| Main uses | Mantle cell lymphoma (MCL), chronic lymphocytic leukemia (CLL)[2][1] |
| Side effects | Headaches, feeling tired, low red blood cells, low platelets, low white blood cells[2] |
| Pregnancy category | |
| Routes of use | By mouth |
| Typical dose | 100 mg BID[4] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a618004 |
| Legal | |
| License data | |
| Legal status | |
| Chemical and physical data | |
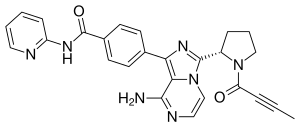
| Formula | C26H23N7O2 |
| Molar mass | 465.517 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Acalabrutinib, sold under the brand name Calquence, is a medication used to treat mantle cell lymphoma (MCL) and chronic lymphocytic leukemia (CLL).[2][1] For CLL it may be used initially or in those who have failed other treatment.[1] It is taken by mouth.[5]
Common side effects include headaches, feeling tired, low red blood cells, low platelets, and low white blood cells.[2] Other side effects may include infection, bleeding, further cancer, and atrial fibrillation.[5] Use in pregnancy may harm the baby.[5] It is a Bruton's tyrosine kinase inhibitor, which slows the build-up of cancerous B cells.[1]
Acalabrutinib was approved for medical use in the United States in 2017 and Europe in 2020.[2][1] In the United Kingdom a month of medication costs the NHS about £5,100 as of 2021.[4] This amount in the United States is about 15,000 USD.[6]
Medical uses
In the European Union, acalabrutinib as monotherapy or in combination with obinutuzumab is indicated for the treatment of adults with previously untreated chronic lymphocytic leukaemia (CLL).[1] It is also indicated for the treatment of adults with chronic lymphocytic leukaemia (CLL) who have received at least one prior therapy.[1]
In the United States, acalabrutinib is indicated for the treatment of adults with mantle cell lymphoma (MCL) who have received at least one prior therapy, and for the treatment of adults with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL).[5]
Dosage
It is taken at a dose of 100 mg twice per day.[4]
Side effects
The most common adverse events were headache, diarrhea and weight gain.[7] Despite the appearance of a greater occurrence of transient headaches, data suggest a preferred advantage of acalabrutinib over ibrutinib due to expected reduced adverse events of skin rash, severe diarrhea, and bleeding risk.[7]
Mechanism of action

In terms of the mechanism of action we find that Acalabrutinib inhibits BTK and that it builds covalent bond with C481.[8]
Society and culture
Legal status
Acalabrutinib was approved for medical use in the United States in 2017,[2] and in the European Union in November 2020.[1]
As of February 2016, acalabrutinib had received orphan drug designation in the United States for mantle cell lymphoma and chronic lymphocytic leukemia (CLL),[9] [10] and was similarly designated as an orphan medicinal product by the European Medicines Agency (EMA) Committee for Orphan Medicinal Products (COMP) for treatment of three indications: CLL/ small lymphocytic lymphoma (SLL), mantle cell lymphoma (MCL), and lymphoplasmacytic lymphoma (Waldenström's macroglobulinaemia, WM).[11][12][13][14] Approval would result in a 10-year period of market exclusivity for the stated indications within Europe.[15]
Economics
It was developed by Acerta Pharma.[16] After promising results for CLL in initial clinical trials,[17] Astra Zeneca purchased a 55% stake in Acerta Pharma for $4 billion in December 2015, with an option to acquire the remaining 45% stake for an additional $3 billion, conditional on approval in both the US and Europe and the establishment of commercial opportunity.[18]
Names
Acalabrutinib is the international nonproprietary name (INN),[19] and the United States Adopted Name (USAN).[20]
Research
Relative to ibrutinib, acalabrutinib demonstrated higher selectivity and inhibition of the targeted activity of BTK, while having a much greater IC50 or otherwise virtually no inhibition on the kinase activities of ITK, EGFR, ERBB2, ERBB4, JAK3, BLK, FGR, FYN, HCK, LCK, LYN, SRC, and YES1.[7] In addition, in platelets treated with ibrutinib, thrombus formation was clearly inhibited while no impact to thrombus formation was identified relative to controls for those treated with acalabrutinib.[7] These findings strongly suggest an improved safety profile of acalabrutinib with minimized adverse effects relative to ibrutinib.[7] In pre-clinical studies, it was shown to be more potent and selective than ibrutinib, the first-in-class BTK inhibitor.[17][7][21]
The interim results of the still on-going[when?] first human phase I/II clinical trial (NCT02029443) with 61 patients for the treatment of relapsed chronic lymphocytic leukemia (CLL) are encouraging, with a 95% overall response rate demonstrating potential to become a best-in-class treatment for CLL.[17] Notably, a 100% response rate was achieved for those people which were positive for the 17p13.1 gene deletion, a subgroup that typically results in a poor response to therapy and expected outcomes.[7]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 1.9 "Calquence EPAR". European Medicines Agency. 20 July 2020. Archived from the original on 17 November 2020. Retrieved 11 November 2020. Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 "Acalabrutinib Monograph for Professionals". Drugs.com. Archived from the original on 4 November 2019. Retrieved 16 March 2019.
- ↑ 3.0 3.1 "Acalabrutinib (Calquence) Use During Pregnancy". Drugs.com. 23 October 2019. Archived from the original on 28 March 2020. Retrieved 28 March 2020.
- ↑ 4.0 4.1 4.2 BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 1010. ISBN 978-0857114105.
- ↑ 5.0 5.1 5.2 5.3 5.4 "Calquence- acalabrutinib capsule, gelatin coated". DailyMed. 22 November 2019. Archived from the original on 23 March 2021. Retrieved 11 November 2020.
- ↑ "Calquence Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 18 January 2021. Retrieved 13 January 2022.
- ↑ 7.0 7.1 7.2 7.3 7.4 7.5 7.6 Wu J, Zhang M, Liu D (March 2016). "Acalabrutinib (ACP-196): a selective second-generation BTK inhibitor". Journal of Hematology & Oncology. 9: 21. doi:10.1186/s13045-016-0250-9. PMC 4784459. PMID 26957112.
- ↑ 8.0 8.1 Vitale, Candida; Gibbons, Jamie Lynn; Ferrajoli, Alessandra (29 December 2021). "Targeted Treatment of Chronic Lymphocytic Leukemia: Clinical Utility of Acalabrutinib". OncoTargets and Therapy. 14: 5507–5519. doi:10.2147/OTT.S303060. Archived from the original on 26 October 2022. Retrieved 24 March 2024.
- ↑ "Acalabrutinib Orphan Drug Designation and Approval". U.S. Food and Drug Administration (FDA). Archived from the original on 23 April 2021. Retrieved 15 April 2020.
- ↑ "Acalabrutinib Orphan Drug Designation and Approval". U.S. Food and Drug Administration (FDA). Archived from the original on 23 April 2021. Retrieved 15 April 2020.
- ↑ "EU/3/16/1624". European Medicines Agency (EMA). 2 May 2016. Archived from the original on 31 July 2020. Retrieved 15 April 2020.
- ↑ "EU/3/16/1625". European Medicines Agency (EMA). 4 May 2016. Archived from the original on 23 April 2021. Retrieved 15 April 2020.
- ↑ "EU/3/16/1626". European Medicines Agency (EMA). 4 May 2016. Archived from the original on 23 April 2021. Retrieved 15 April 2020.
- ↑ "azn201602256k.htm". www.sec.gov. Archived from the original on 2016-11-21. Retrieved 2016-11-21.
- ↑ House DW (2016-02-25). "AstraZeneca and Acerta Pharma's acalabrutinib tagged an Orphan Drug in Europe for three indications". Seeking Alpha. Archived from the original on 2016-11-21. Retrieved 2016-11-21.
- ↑ "AstraZeneca to buy Acerta for blood cancer drug". www.rsc.org. Chemistry World - Royal Society of Chemistry. Archived from the original on 24 December 2015. Retrieved 24 December 2015.
- ↑ 17.0 17.1 17.2 Byrd JC, Harrington B, O'Brien S, Jones JA, Schuh A, Devereux S, et al. (January 2016). "Acalabrutinib (ACP-196) in Relapsed Chronic Lymphocytic Leukemia". The New England Journal of Medicine. 374 (4): 323–32. doi:10.1056/NEJMoa1509981. PMC 4862586. PMID 26641137.
- ↑ Walker I, Roland D (2015-12-17). "AstraZeneca to Buy Stake in Acerta Pharma". Wall Street Journal. ISSN 0099-9660. Archived from the original on 2016-11-20. Retrieved 2016-11-19.
- ↑ World Health Organization (2016). "International nonproprietary names for pharmaceutical substances (INN): recommended INN: list 75". WHO Drug Information. 30 (1): 94. hdl:10665/331046.
- ↑ "Acalabrutinib" (PDF). 27 January 2016. Archived (PDF) from the original on 13 November 2020. Retrieved 2 November 2021.
- ↑ Wu J, Zhang M, Liu D (March 2016). "Acalabrutinib (ACP-196): a selective second-generation BTK inhibitor". Journal of Hematology & Oncology. 9 (1): 21. doi:10.1186/s13045-016-0250-9. PMC 4784459. PMID 26957112.
External links
| External sites: | |
|---|---|
| Identifiers: |
|
- "Acalabrutinib". NCI Drug Dictionary. National Cancer Institute. Archived from the original on 2020-11-13. Retrieved 2021-11-02.
- "Acalabrutinib". National Cancer Institute. Archived from the original on 2021-07-09. Retrieved 2021-11-02.
- Pages using duplicate arguments in template calls
- Drugs with non-standard legal status
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without UNII source
- Drugs missing an ATC code
- Articles containing unverified chemical infoboxes
- All articles with vague or ambiguous time
- Vague or ambiguous time from March 2020
- Articles with invalid date parameter in template
- Antineoplastic drugs
- Human proteins
- Tyrosine kinase inhibitors
- AstraZeneca brands
- Alkyne derivatives
- Orphan drugs
- RTT