Evinacumab
| Monoclonal antibody | |
|---|---|
| Type | Whole antibody |
| Source | Human |
| Target | Angiopoietin-like 3 (ANGPTL3) |
| Names | |
| Trade names | Evkeeza |
| Other names | REGN1500, evinacumab-dgnb |
| Clinical data | |
| Main uses | Homozygous familial hypercholesterolemia (HoFH)[1] |
| Side effects | Influenza-like illness, dizziness, runny nose, nausea[1] |
| Routes of use | Intravenous |
| Typical dose | 15 mg/kg q 4 weeks[1] |
| External links | |
| AHFS/Drugs.com | Monograph |
| Legal | |
| License data |
|
| Legal status | |
| Chemical and physical data | |
| Formula | C6480H9992N1716O2042S46 |
| Molar mass | 146083.95 g·mol−1 |
Evinacumab, sold under the brand name Evkeeza, is a medication used to treat homozygous familial hypercholesterolemia (HoFH).[1] It is used in those who are at least 12 years old; however, effects on heart disease and life expectancy is unclear as of 2022.[1] It is given by injection into a vein.[1]
Common side effects include the influenza-like illness, dizziness, runny nose, and nausea.[1] Other side effects may include anaphylaxis.[1] Use in pregnancy may harm the baby.[1] It is a monoclonal antibody that binds to and blocks angiopoietin-like protein 3 (ANGPTL3), resulting in faster break down of fats.[1][2]
Evinacumab was approved for medical use in the United States and Europe in 2021.[1][2] In the United States it costs about 450,000 USD per year as of 2022.[4]
Medical uses
Over 24 weeks, in those with homozygous familial hypercholesterolemia (HoFH), it reduced LDL cholesterol levels by almost half compared to about 2% seen with a placebo.[2]
Dosage
It is given at a dose of 15 mg/kg every 4 weeks.[1]
Mechanism of action
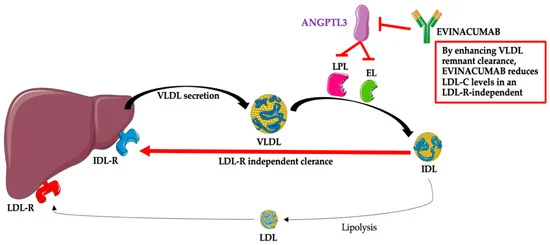
In terms of the mechanism of action of Evinacumab we find that it works via inhibiting ANGPTL3 which in turn reduces LDL-C and triglycerides levels. Hence effective against familial hypercholesterolemia.[5]
History
The effectiveness and safety of evinacumab were evaluated in a double-blind, randomized, placebo-controlled, 24-week trial enrolling 65 participants with homozygous familial hypercholesterolemia (HoFH).[3] In the trial, 43 participants received 15 mg/kg of evinacumab every four weeks and 22 participants received the placebo.[3] Participants were taking other lipid-lowering therapies as well.[3]
The primary measure of effectiveness was the percent change in low-density lipoprotein (LDL-C) from the beginning of treatment to week 24.[3] At week 24, participants receiving evinacumab had an average 47% decrease in LDL-C while participants on the placebo had an average 2% increase.[3]
The U.S. Food and Drug Administration (FDA) granted the application for evinacumab orphan drug, breakthrough therapy, and priority review designations.[3] The FDA granted approval of Evkeeza to Regeneron Pharmaceuticals, Inc.[3] Regeneron invented evinacumab using the company's genetically-engineered mouse platform endowed with a genetically-humanized immune system to produce fully-human monoclonal antibodies.[6]
Society and culture
Legal status
On 22 April 2021, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) adopted a positive opinion, recommending the granting of a marketing authorization under exceptional circumstances for the medicinal product Evkeeza, intended for the treatment of adult and adolescent patients aged 12 years and older with homozygous familial hypercholesterolaemia (HoFH).[7] The applicant for this medicinal product is Regeneron Ireland Designated Activity Company (DAC).[7] Evinacumab was approved for medical use in the European Union in June 2021.[2]
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 "Evkeeza- evinacumab injection, solution, concentrate". DailyMed. Archived from the original on 14 September 2021. Retrieved 14 September 2021.
- ↑ 2.0 2.1 2.2 2.3 2.4 "Evkeeza EPAR". European Medicines Agency (EMA). 21 April 2021. Archived from the original on 19 December 2021. Retrieved 18 December 2021.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 3.7 "FDA approves add-on therapy for patients with genetic form of severely". U.S. Food and Drug Administration (FDA). 11 February 2021. Archived from the original on 12 February 2021. Retrieved 12 February 2021.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "Evinacumab". SPS - Specialist Pharmacy Service. 21 September 2018. Archived from the original on 3 March 2022. Retrieved 31 October 2022.
- ↑ 5.0 5.1 Sosnowska, Bożena; Adach, Weronika; Surma, Stanisław; Rosenson, Robert S.; Banach, Maciej (January 2023). "Evinacumab, an ANGPTL3 Inhibitor, in the Treatment of Dyslipidemia". Journal of Clinical Medicine. 12 (1): 168. doi:10.3390/jcm12010168. ISSN 2077-0383. Archived from the original on 2022-12-25. Retrieved 2023-11-20.
- ↑ "FDA Accepts Evinacumab Biologics License Application for Priority Review as a Treatment for Patients with HoFH, an Ultra-rare Inherited Form of High Cholesterol | Regeneron Pharmaceuticals Inc". investor.regeneron.com. Archived from the original on 2021-08-30. Retrieved 2021-08-30.
- ↑ 7.0 7.1 "Evkeeza: Pending EC decision". European Medicines Agency. 23 April 2021. Archived from the original on 23 April 2021. Retrieved 23 April 2021.
Further reading
- Dewey FE, Gusarova V, Dunbar RL, O'Dushlaine C, Schurmann C, Gottesman O, et al. (July 2017). "Genetic and Pharmacologic Inactivation of ANGPTL3 and Cardiovascular Disease". N Engl J Med. 377 (3): 211–221. doi:10.1056/NEJMoa1612790. PMC 5800308. PMID 28538136.
External links
| External sites: | |
|---|---|
| Identifiers: |
|
- Pages using duplicate arguments in template calls
- Wikipedia articles incorporating the PD-notice template
- Drugs with non-standard legal status
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Drugs missing an ATC code
- Articles containing unverified chemical infoboxes
- Drugs that are a monoclonal antibody
- Chemicals that do not have a ChemSpider ID assigned
- Breakthrough therapy
- Monoclonal antibodies
- Orphan drugs
- RTT