Everolimus
 | |
 | |
| Names | |
|---|---|
| Pronunciation | Everolimus /ˌɛvəˈroʊləməs/ |
| Trade names | Afinitor, Zortress, others |
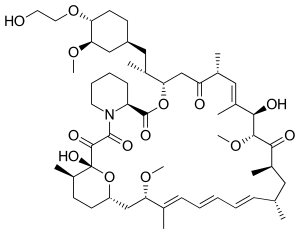
| Other names | 42-O-(2-hydroxyethyl)rapamycin, RAD001 |
| |
| Clinical data | |
| Drug class | mTOR inhibitor, kinase inhibitor[1] |
| Pregnancy category | |
| Routes of use | By mouth |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a609032 |
| Legal | |
| License data |
|
| Legal status |
|
| Pharmacokinetics | |
| Elimination half-life | ~30 hours[3] |
| Chemical and physical data | |
| Formula | C53H83NO14 |
| Molar mass | 958.240 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Everolimus is a medication used to prevent rejection after an organ transplant and to treat certain types of cancer.[1] This includes after kidney, heart, and liver transplant.[4] It may be used for breast, kidney, and neuroendocrine tumors.[5] It is taken by mouth.[1]
Common side effects include mouth inflammation, respiratory tract infection, tiredness, vomiting, and diarrhea.[1] Other side effects may include hair loss, low white blood cells, bleeding, kidney problems, blood clotting, and heart failure.[4] It is likely that use during pregnancy would harm the baby.[6] It is a derivative of sirolimus and works similarly; by blocking mammalian target of rapamycin (mTOR).[5][7]
Everolimus was approved for medical use in the United States and Europe in 2009.[1][5] It is available as a generic medication.[4] It is on the World Health Organization's List of Essential Medicines.[8] In the United Kingdom a month of treatment post transplant costs about £450 while for certain types of cancer can be £2,900 as of 2020.[4] A similar amount to prevent transplant rejection costs about 450 USD in the United States as of 2021.[9] A generic version was approved in 2021 in the USA.[10]
Medical uses
Everolimus is approved for various conditions:
- Advanced kidney cancer (US FDA approved in March 2009)[11]
- Prevention of organ rejection after renal transplant(US FDA April 2010)[12]
- Subependymal giant cell astrocytoma (SEGA) associated with tuberous sclerosis (TS) in patients who are not suitable for surgical intervention (US FDA October 2010)[13]
- Progressive or metastatic pancreatic neuroendocrine tumors not surgically removable (May 2011)[14]
- Breast cancer in post-menopausal women with advanced hormone-receptor positive, HER2-negative type cancer, in conjunction with exemestane (US FDA July 2012)[15]
- Prevention of organ rejection after liver transplant(Feb 2013)
- Progressive, well-differentiated non-functional, neuroendocrine tumors (NET) of gastrointestinal (GI) or lung origin with unresectable, locally advanced or metastatic disease (US FDA February 2016).[16]
- Tuberous sclerosis complex-associated partial-onset seizures for adult and children aged 2 years and older. (US FDA April 2018).[17]
Heart transplant
Everolimus may have a role in heart transplantation, as it has been shown to reduce chronic allograft vasculopathy in such transplants. It also may have a similar role to sirolimus in kidney and other transplants.[18]
Vascular stents
Everolimus is used in drug-eluting coronary stents as an immunosuppressant to prevent restenosis.
Dosage
To prevent transplant rejection doses of 750 ug to 1 mg twice per day may be used.[4]
For neuroendocrine tumors a dose of 10 mg per day may be used.[4]
Side effects
A trial using 10 mg/day in patients with NETs of GI or lung origin reported "Everolimus was discontinued for adverse reactions in 29% of patients and dose reduction or delay was required in 70% of everolimus-treated patients. Serious adverse reactions occurred in 42% of everolimus-treated patients and included 3 fatal events (cardiac failure, respiratory failure, and septic shock). The most common adverse reactions (incidence greater than or equal to 30%) were stomatitis, infections, diarrhea, peripheral edema, fatigue and rash. The most common blood abnormalities found (incidence greater than or equal to 50%) were anemia, hypercholesterolemia, lymphopenia, elevated aspartate transaminase (AST) and fasting hyperglycemia.".[16]
Mechanism
Compared with the parent compound rapamycin, everolimus is more water-soluble.[19] Compared to rapamycin, everolimus is more selective for the mTORC1 protein complex, with little impact on the mTORC2 complex.[20] This can lead to a hyper-activation of the kinase AKT via inhibition on the mTORC1 negative feedback loop, while not inhibiting the mTORC2 positive feedback to AKT. This AKT elevation can lead to longer survival in some cell types. Thus, everolimus has important effects on cell growth, cell proliferation and cell survival.
mTORC1 inhibition by everolimus has been shown to normalize tumor blood vessels, to increase tumor-infiltrating lymphocytes, and to improve adoptive cell transfer therapy.[21]
Additionally, mTORC2 is believed to play an important role in glucose metabolism and the immune system, suggesting that selective inhibition of mTORC1 by drugs such as everolimus could achieve many of the benefits of rapamycin without the associated glucose intolerance and immunosuppression.[20]
TSC1 and TSC2, the genes involved in tuberous sclerosis, act as tumor suppressor genes by regulating mTORC1 activity. Thus, either the loss or inactivation of one of these genes lead to the activation of mTORC1.[22]
Everolimus binds to its protein receptor FKBP12, which directly interacts with mTORC1, inhibiting its downstream signaling. As a consequence, mRNAs that code for proteins implicated in the cell cycle and in the glycolysis process are impaired or altered, and tumor growth is inhibited.[22]
Society and culture
It is marketed by Novartis under the trade names Zortress (USA) and Certican (European Union and other countries) in transplantation medicine, and as Afinitor (general tumours) and Votubia (tumours as a result of TSC) in oncology. Everolimus is also available from Biocon, with the brand name Evertor.
UK National Health Service
NHS England has been criticised for delays in deciding on a policy for the prescription of everolimus in the treatment of tuberous sclerosis. 20 doctors addressed a letter to the board in support of the charity Tuberous Sclerosis Association saying " around 32 patients with critical need, whose doctors believe everolimus treatment is their best or only option, have no hope of access to funding. Most have been waiting many months. Approximately half of these patients are at imminent risk of a catastrophic event (renal bleed or kidney failure) with a high risk of preventable death."[23] In May 2015 it was reported that Luke Henry and Stephanie Rudwick, the parents of a child were tuberous sclerosis were trying to sell their home in Brighton to raise £30,000 to pay for treatment for their daughter who has tumours on her brain, kidneys and liver with up to 50 epileptic fits a day.[24]
Research
As of October 2010[update], Phase III trials are under way in gastric cancer, hepatocellular carcinoma, and lymphoma.[25] The experimental use of everolimus in refractory chronic graft-versus-host disease was reported in 2012.[26]
A phase 2a randomized, placebo-controlled everolimus clinical trial published in 2014 showed that everolimus improved the response to an influenza vaccine by 20% in healthy elderly volunteers.[27] A phase 2a randomized, placebo-controlled clinical trial published in 2018 showed that everolimus in combination with dactolisib decreased the rate of reported infections in an elderly population.[27]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 "Everolimus Monograph for Professionals". Drugs.com. Archived from the original on 17 April 2021. Retrieved 23 July 2021.
- ↑ 2.0 2.1 Use During Pregnancy and Breastfeeding
- ↑ Formica RN, Lorber KM, Friedman AL, Bia MJ, Lakkis F, Smith JD, Lorber MI (March 2004). "The evolving experience using everolimus in clinical transplantation". Transplantation Proceedings. 36 (2 Suppl): 495S–499S. doi:10.1016/j.transproceed.2004.01.015. PMID 15041395.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 BNF (80 ed.). BMJ Group and the Pharmaceutical Press. September 2020 – March 2021. p. 1034. ISBN 978-0-85711-369-6.
{{cite book}}: CS1 maint: date format (link) - ↑ 5.0 5.1 5.2 "Afinitor". Archived from the original on 8 April 2021. Retrieved 23 July 2021.
- ↑ "Everolimus Use During Pregnancy". Drugs.com. Archived from the original on 17 April 2021. Retrieved 23 July 2021.
- ↑ Wheeler, Derek S.; Wong, Hector R. (2007). Pediatric Critical Care Medicine: Basic Science And Clinical Evidence. Springer Science & Business Media. p. 1391. ISBN 978-1-84628-463-2. Archived from the original on 2021-08-28. Retrieved 2021-07-23.
- ↑ World Health Organization (2021). World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. hdl:10665/345533. WHO/MHP/HPS/EML/2021.02.
- ↑ "Everolimus Prices, Coupons & Savings Tips - GoodRx". GoodRx. Retrieved 23 July 2021.
- ↑ Research, Center for Drug Evaluation and (10 February 2022). "2021 First Generic Drug Approvals". FDA. Archived from the original on 21 June 2022. Retrieved 22 October 2022.
- ↑ "Afinitor approved in US as first treatment for patients with advanced kidney cancer after failure of either sunitinib or sorafenib" (Press release). Novartis. 2009-03-30. Archived from the original on 2009-04-03. Retrieved 6 April 2009.
- ↑ "Novartis receives US FDA approval for Zortress (everolimus) to prevent organ rejection in adult kidney transplant recipients" (Press release). Novartis. 2010-04-22. Archived from the original on 25 April 2010. Retrieved 26 April 2010.
- ↑ "Novartis' Afinitor Cleared by FDA for Treating SEGA Tumors in Tuberous Sclerosis". 1 Nov 2010. Archived from the original on 7 March 2016. Retrieved 5 July 2021.
- ↑ "Archive copy". Archived from the original on 2014-08-01. Retrieved 2021-07-05.
{{cite web}}: CS1 maint: archived copy as title (link) - ↑ "US FDA approves Novartis drug Afinitor for breast cancer". Reuters. 20 Jul 2012. Archived from the original on 11 March 2016. Retrieved 5 July 2021.
- ↑ 16.0 16.1 "Everolimus (Afinitor). Feb 2016". Archived from the original on 2019-04-23. Retrieved 2021-07-05.
- ↑ "Everolimus (Afinitor). April 2018". Archived from the original on 2019-04-23. Retrieved 2021-07-05.
- ↑ Eisen HJ, Tuzcu EM, Dorent R, Kobashigawa J, Mancini D, Valantine-von Kaeppler HA, Starling RC, Sørensen K, Hummel M, Lind JM, Abeywickrama KH, Bernhardt P (August 2003). "Everolimus for the prevention of allograft rejection and vasculopathy in cardiac-transplant recipients". The New England Journal of Medicine. 349 (9): 847–58. doi:10.1056/NEJMoa022171. PMID 12944570.
- ↑ Magaway C, Kim E, Jacinto E (2019). "Targeting mTOR and Metabolism in Cancer: Lessons and Innovations". Cells. 8 (12): 1584. doi:10.3390/cells8121584. PMC 6952948. PMID 31817676.
- ↑ 20.0 20.1 Arriola Apelo SI, Neuman JC, Baar EL, Syed FA, Cummings NE, Brar HK, Pumper CP, Kimple ME, Lamming DW (February 2016). "Alternative rapamycin treatment regimens mitigate the impact of rapamycin on glucose homeostasis and the immune system". Aging Cell. 15 (1): 28–38. doi:10.1111/acel.12405. PMC 4717280. PMID 26463117.
- ↑ Wang S, Raybuck A, Shiuan E, Jin J (2020). "Selective inhibition of mTORC1 in tumor vessels increases antitumor immunity". JCI Insight. 5 (15): e139237. doi:10.1172/jci.insight.139237. PMC 7455083. PMID 32759497.
- ↑ 22.0 22.1 "Archived copy". Archived from the original on 2014-03-08. Retrieved 2014-02-26.
{{cite web}}: CS1 maint: archived copy as title (link) - ↑ Lintern, Shaun (14 April 2015). "Policy delays risk 'preventable deaths', doctors warn NHS England". Health Service Journal. Archived from the original on 27 April 2015. Retrieved 20 April 2015.
- ↑ "Couple forced to sell home after NHS refuse to fund daughter's treatment for rare illness". Daily Express. 11 May 2015. Archived from the original on 12 May 2015. Retrieved 12 May 2015.
- ↑ "Archive copy". Archived from the original on 2016-03-07. Retrieved 2021-07-05.
{{cite web}}: CS1 maint: archived copy as title (link) - ↑ Lutz M, Kapp M, Grigoleit GU, Stuhler G, Einsele H, Mielke S (April 2012). "Salvage therapy with everolimus improves quality of life in patients with refractory chronic graft-versus-host disease" (PDF). Bone Marrow Transplant. 47 (S1): S410–S411. Archived (PDF) from the original on 2016-08-11. Retrieved 2021-07-05.
- ↑ 27.0 27.1 Zhavoronkov A (2020). "Geroprotective and senoremediative strategies to reduce the comorbidity, infection rates, severity, and lethality in gerophilic and gerolavic infections". Aging. 12 (8): 6492–6510. doi:10.18632/aging.102988. PMC 7202545. PMID 32229705.
External links
| External sites: | |
|---|---|
| Identifiers: |
|
- Sedrani R, Cottens S, Kallen J, Schuler W (August 1998). "Chemical modification of rapamycin: the discovery of SDZ RAD". Transplantation Proceedings. 30 (5): 2192–4. doi:10.1016/S0041-1345(98)00587-9. PMID 9723437.
- Pages using duplicate arguments in template calls
- CS1 maint: date format
- CS1 maint: archived copy as title
- Drugs with non-standard legal status
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without UNII source
- Drugs missing an ATC code
- Drug has EMA link
- Drugboxes which contain changes to verified fields
- Drugboxes which contain changes to watched fields
- Articles containing potentially dated statements from October 2010
- Articles with invalid date parameter in template
- All articles containing potentially dated statements
- Articles with changed EBI identifier
- Antineoplastic drugs
- Immunosuppressants
- Macrolides
- Novartis brands
- Polyenes
- RTT
- World Health Organization essential medicines