Teprotumumab
| Monoclonal antibody | |
|---|---|
| Type | Whole antibody |
| Source | Human |
| Target | IGF-1R[1] |
| Names | |
| Trade names | Tepezza |
| Other names | Teprotumumab-trbw, RG-1507 |
| Clinical data | |
| Drug class | Monoclonal antibody[1] |
| Main uses | Thyroid eye disease (TED)[1] |
| Side effects | Muscle spasm, nausea, hair loss, diarrhea, fatigue, high blood sugar, hearing loss, dry skin, altered taste, headache[2] |
| Routes of use | Intravenous |
| External links | |
| AHFS/Drugs.com | Monograph |
| Legal | |
| License data |
|
| Legal status |
|
| Chemical and physical data | |
| Formula | C6476H10012N1748O2000S40 |
| Molar mass | 145639.97 g·mol−1 |
Teprotumumab, sold under the brand name Tepezza, is a medication used to treat thyroid eye disease (TED).[1] It decreases eye protrusion (proptosis).[2] It is given by slow injection into a vein.[1]
Common side effects include muscle spasm, nausea, hair loss, diarrhea, fatigue, high blood sugar, hearing loss, dry skin, altered taste, and headache.[2] Other side effects may include Infusion reactions.[1] It should not be used during or for the six months prior to pregnant.[2] It is a human monoclonal antibody which binds to insulin-like growth factor-1 receptor (IGF-1R) thereby preventing its activation.[1]
Teprotumumab was approved for medical use in the United States in 2020.[1] As of 2021 it is not approved in Europe.[3] In the United States it costs about 15,600 USD per 500 mg vial as of 2021.[4]
Medical use
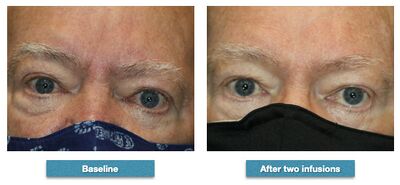
In Graves' ophthalmopathy teprotumumab was more effective than placebo.[5] 82.9 percent of teprotumumab patients compared to 9.5 percent of placebo patients improved 2 mm or more reduction in proptosis. All secondary endpoints were also met and the safety profile was consistent with the Phase II study of teprotumumab in TED.[6]
Dosage
It is generally give as 8 doses with the first of 10 mg/kg followed every three weeks at a dose of 20 mg/kg.[1]
History
Teprotumumab-trbw was approved for use in the United States in January 2020, for the treatment of adults with thyroid eye disease.[2][7]
On 10 July 2019, Horizon submitted a Biologics License Application (BLA) to the FDA for teprotumumab for the treatment of active thyroid eye disease (TED). Horizon requested priority review for the application - if so granted (FDA has a 60-day review period to decide) it would result in a max. 6 month review process.[8]
Teprotumumab was first investigated for the treatment of solid and hematologic tumors, including breast cancer, Hodgkin's and non-Hodgkin's lymphoma, non-small cell lung cancer and sarcoma.[9] Although results of Phase I and early Phase II trials showed promise, research for these indications were discontinued in 2009, by Roche. Phase II trials still in progress were allowed to complete, as the development was halted due to business prioritization rather than safety concerns.
Teprotumumab was subsequently licensed to River Vision Development Corporation in 2012, for research in the treatment of ophthalmic conditions. Horizon Pharma (now Horizon Therapeutics, from hereon Horizon) acquired RVDC in 2017, and will continue clinical trials.[10] It is in Phase III trials for Graves' ophthalmopathy (also known as thyroid eye disease (TED)) and Phase I for diabetic macular edema.[11] It was granted Breakthrough Therapy, Orphan Drug Status and Fast Track designations by the FDA for Graves' ophthalmopathy.[10]
Teprotumumab-trbw was approved based on the results of two clinical trials (Trial 1/ NCT01868997 and Trial 2/ NCT03298867) of 170 subjects with active thyroid eye disease who were randomized to either receive teprotumumab-trbw or a placebo.[2][12] Of the subjects who were administered Tepezza, 71% in Study 1 and 83% in Study 2 demonstrated a greater than two millimeter reduction in proptosis (eye protrusion) as compared to 20% and 10% of subjects who received placebo, respectively.[2] The trials were conducted at 28 sites in Europe and United States.[12]
The U.S. Food and Drug Administration (FDA) granted the application for teprotumumab-trbw fast track designation, breakthrough therapy designation, priority review designation, and orphan drug designation.[2] The FDA granted the approval of Tepezza to Horizon Therapeutics Ireland DAC.[2]
The U.S. Food and Drug Administration (FDA) considers it to be a first-in-class medication.[13]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 "Teprotumumab-trbw Monograph for Professionals". Drugs.com. Retrieved 26 September 2021.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 2.8 "FDA approves first treatment for thyroid eye disease". U.S. Food and Drug Administration (FDA) (Press release). 21 January 2020. Archived from the original on 28 August 2020. Retrieved 21 January 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "Teprotumumab". SPS - Specialist Pharmacy Service. 21 March 2018. Archived from the original on 27 September 2021. Retrieved 26 September 2021.
- ↑ "Tepezza Prices, Coupons & Patient Assistance Programs". Drugs.com. Retrieved 26 September 2021.
- ↑ Smith TJ, Kahaly GJ, Ezra DG, Fleming JC, Dailey RA, Tang RA, et al. (May 2017). "Teprotumumab for Thyroid-Associated Ophthalmopathy". The New England Journal of Medicine. 376 (18): 1748–1761. doi:10.1056/NEJMoa1614949. PMC 5718164. PMID 28467880.
- ↑ "Horizon Pharma plc Announces Phase 3 Confirmatory Trial Evaluating Teprotumumab (OPTIC) for the Treatment of Active Thyroid Eye Disease (TED) Met Primary and All Secondary Endpoints". Horizon Pharma plc. Archived from the original on 28 May 2019. Retrieved 22 March 2019.
- ↑ "Teprotumumab-trbw: FDA-Approved Drugs". U.S. Food and Drug Administration (FDA). Archived from the original on 18 January 2021. Retrieved 28 January 2020.
- ↑ "Horizon Therapeutics plc Submits Teprotumumab Biologics License Application (BLA) for the Treatment of Active Thyroid Eye Disease (TED)". Horizon Therapeutics plc. Archived from the original on 26 January 2021. Retrieved 27 August 2019.
- ↑ Clinical trial number NCT01868997 for "Teprotumumab (RV 001) Treatment in Patients With Active Thyroid Eye Disease" at ClinicalTrials.gov
- ↑ 10.0 10.1 "Teprotumumab". Genmab A/S. Archived from the original on 22 May 2020. Retrieved 1 July 2021.
- ↑ "Teprotumumab - Horizon Therapeutics plc". Adis International Ltd. Springer Nature Switzerland AG. Archived from the original on 12 March 2020. Retrieved 1 July 2021.
- ↑ 12.0 12.1 "Drug Trial Snapshot: Tepezza". U.S. Food and Drug Administration (FDA). 29 January 2020. Archived from the original on 25 February 2020. Retrieved 29 January 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "New Drug Therapy Approvals 2020". U.S. Food and Drug Administration (FDA). 31 December 2020. Archived from the original on 18 January 2021. Retrieved 17 January 2021.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
External links
| External sites: | |
|---|---|
| Identifiers: |
|
- Pages using duplicate arguments in template calls
- Wikipedia articles incorporating the PD-notice template
- Use dmy dates from January 2020
- Articles with invalid date parameter in template
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Drugs missing an ATC code
- Drugboxes which contain changes to verified fields
- Drugboxes which contain changes to watched fields
- Drugs that are a monoclonal antibody
- Drugs not assigned an ATC code
- Articles with changed CASNo identifier
- Chemicals that do not have a ChemSpider ID assigned
- Articles with changed KEGG identifier
- Breakthrough therapy
- Hoffmann-La Roche brands
- Orphan drugs
- RTT