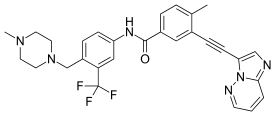
Ponatinib
 | |
 | |
| Names | |
|---|---|
| Pronunciation | /poʊˈnætɪnɪb/ poh-NAT-i-nib Iclusig: /aɪˈkluːsɪɡ/ eye-KLOO-sig |
| Trade names | Iclusig |
| Other names | AP24534 |
| |
| Clinical data | |
| Drug class | Tyrosine-kinase inhibitor[1] |
| Main uses | Chronic myeloid leukemia (CML), acute lymphoblastic leukemia (ALL)[1] |
| Side effects | Blood clost, pneumonia, pancreatitis, fever, abdominal pain, heart attack, atrial fibrillation, low platelets, low neutrophils, stroke, kidney problems, heart failure[2] |
| Pregnancy category |
|
| Routes of use | By mouth (tablets) |
| Typical dose | 45 mg OD[1] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a613029 |
| Legal | |
| License data | |
| Legal status |
|
| Pharmacokinetics | |
| Bioavailability | Unknown |
| Protein binding | >99% (in vitro) |
| Metabolism | Liver (CYP3A4, 2C8, 2D6, 3A5) |
| Elimination half-life | 12–66 hours |
| Excretion | Feces (87%), urine (5%)[3] |
| Chemical and physical data | |
| Formula | C29H27F3N6O |
| Molar mass | 532.571 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Ponatinib, sold under the brand name Iclusig, is a medication used to treat chronic myeloid leukemia (CML) and acute lymphoblastic leukemia (ALL) which is Philadelphia chromosome–positive (Ph+).[1] It is used when other treatments have failed.[1] It is taken by mouth.[1]
Common side effects include pneumonia, pancreatitis, fever, abdominal pain, heart attack, atrial fibrillation, low platelets, low neutrophils, stroke, kidney problems, and heart failure.[2] Serious blood clots occur in arteries in about 25% of people and veins in about 5% of people.[2] It is a tyrosine-kinase inhibitor which blocks Bcr-Abl.[1][2]
Ponatinib was approved for medical use in the United States in 2012 and Europe in 2013.[1][2] In the United Kingdom it costs the NHS about £5,100 per month.[4] This amount in the United States is about 19,000 USD.[5]
Medical uses
Ponatinib is used for people with resistant or intolerant CML and Ph+ ALL, based on results of the PACE phase II trial reported days earlier at the annual ASH meeting.[6] Because the approval was under the FDA Accelerated Approval program the applicant was required to carry out additional studies. Based on these additional studies, the FDA granted in 2016 full approval and updated the label to include patients with chronic phase, accelerated phase, or blast phase chronic myeloid leukemia and Philadelphia chromosome-positive acute lymphoblastic leukemia for whom no other tyrosine kinase inhibitor therapy is indicated. Approval was also granted for T315I-positive and T315I-positive Philadelphia chromosome positive acute lymphoblastic leukemia.[7]
Dosage
It is generally taken at a dose of 45 mg per day.[1] Lower doses may be used in those with significant side effects.[1]
Side effects
The United States Food and Drug Administration issued a partial clinical hold on new trial enrollment for ponatinib on October 9, 2013 due to an increased number of blood clots observed in patients taking the drug.[8] The EPIC trial was later canceled on October 18.[9] Subsequent studies of 449 patients treated during 4 years with ponatinib for chronic phase chronic myelogenous leukemia found the following adverse reactions. 150 Patients experienced cardiac vascular (21% of patients), peripheral vascular (12%), and cerebrovascular (9%) arterial occlusive events. Venous thromboembolic events occurred in 6% of patients. The most common all-grade adverse events included hypertension (69%), rash (63%), abdominal pain (48%), fatigue (47%), headache (43%), arterial ischemia (42%), dry skin (42%), constipation (41%), arthralgia (32%), nausea (28%), pyrexia (26%), peripheral neuropathy (24%), myalgia (24%), pain in extremity (23%), back pain (21%), and diarrhea (20%). In addition, there have been reported cases of the posterior reversible encephalopathy syndrome.[10]
Mechanism of action

The primary target for ponatinib is BCR-ABL, an abnormal tyrosine kinase that is the hallmark of CML and Ph+ ALL. CML is characterized by an excessive and unregulated production of white blood cells by the bone marrow due to a genetic abnormality that produces the BCR-ABL protein. After a chronic phase of production of too many white blood cells, CML typically evolves to more aggressive phases such as accelerated or blast crisis. Ph+ ALL is a subtype of acute lymphoblastic leukemia that carries the Ph+ chromosome that produces BCR-ABL. It has a more aggressive course than CML and is often treated with a combination of chemotherapy and tyrosine kinase inhibitors. Because both of these diseases express the BCR-ABL protein, this would render them potentially susceptible to treatment with ponatinib. BCR-ABL is detected in 95% of patients with CML.[citation needed]
Patients with CML currently receive front line therapies nilotinib and/or dasatinib though 22−33% of patients discontinue therapy by two years due to adverse events, treatment failure and other causes.[citation needed]
History
Ponatinib was designed using ARIAD's computational and structure-based drug design platform to inhibit the enzymatic activity of BCR-ABL with very high potency and broad specificity. Ponatinib was intended to target not only native BCR-ABL, but also its isoforms that carry mutations that confer resistance to treatment with existing tyrosine kinase inhibitors, including especially the T315I mutation for which no effective therapy exists.[12]
The road to discovery is linked to AP23464, one of the first of Ariad's ATP competitive dual Src/Abl inhibitors. AP23464 was identified using structure base drug design and focused synthetic libraries of trisubstituted purine analogs. The substance potently inhibits Src and Bcr-Abl kinases including many common imatinib-resistant Bcr-Abl mutations. AP23464 does not inhibit the T315I mutation, however, whereas ponatinib does.
Society and culture
Cost
Sales were temporarily suspended from October to December of 2013 due to the risk of blood clots and severe narrowing of blood vessels.[13][14] In the US it can cost $138,000 a year.[15][16]
[US] Oncologists have expressed concerns that people cannot afford the cost of $138,000 a year, which makes it one of the most expensive drugs in medicine, and [in their view] far more expensive than what is needed to pay the development costs.[15][16]
In 2015 ponatinib is available in England for the treatment of CML (chronic phase, accelerated phase or blast phase) and Ph+ ALL in patients with documented T315I mutation under the Cancer Drugs Fund,[17] and has not been appraised by the National Institute for Health and Care Excellence (NICE), who noted the small expected patient population.[18] NICE estimated that ponatinib would cost approximately £61,000 per year, but the price paid under the Cancer Drugs Fund is confidential and may be different.
Research
In 2010 ARIAD announced result from a Phase I study of ponatinib in patients with resistant and refractory chronic myeloid leukemia (CML) and Philadelphia-positive acute lymphoblastic leukemia (Ph+ ALL). The study demonstrated that in chronic-phase CML patients treated with ponatinib, 66 percent of patients in the trial achieved a major cytogenetic response, including 100 percent of patients who also had a T315I mutation.[citation needed]
The PACE (Ponatinib Ph+ ALL and CML Evaluation) pivotal phase II trial started enrolling patients in September 2010 and is designed to provide definitive clinical data for regulatory approval in this setting. Good results were reported in December 2012.[6][19]
The EPIC (Evaluation of Ponatinib versus Imatinib in CML) phase-III trial began in June 2012 [20] and was halted[clarification needed][9] on October 18, 2013.
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 1.9 "Ponatinib Monograph for Professionals". Drugs.com. Archived from the original on 6 March 2016. Retrieved 28 October 2021.
- ↑ 2.0 2.1 2.2 2.3 2.4 "Iclusig". Archived from the original on 13 August 2021. Retrieved 28 October 2021.
- ↑ "Iclusig (ponatinib) Tablets, for Oral Use. Full Prescribing Information". ARIAD Pharmaceuticals, Inc. 26 Landsdowne Street, Cambridge, MA 02139-4234. Archived from the original on 17 October 2018. Retrieved 2 October 2016.
- ↑ BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 1041. ISBN 978-0857114105.
- ↑ "Iclusig Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 16 April 2021. Retrieved 28 October 2021.
- ↑ 6.0 6.1 Gever J (Dec 14, 2012). "Ponatinib Wins Early FDA Nod". Oncology/Hematology. MedPageToday.com. Archived from the original on January 27, 2021. Retrieved August 11, 2021.
- ↑ "FDA Grants Ponatinib Full Approval for Rare Leukemias". Archived from the original on 2020-03-22. Retrieved 2021-08-11.
- ↑ Carroll J. "UPDATED: Ariad hammered on toxicity concerns for leukemia drug Iclusig". FierceBiotech. Archived from the original on 2016-03-03. Retrieved 2021-08-11.
- ↑ 9.0 9.1 "ARIAD Announces Discontinuation of the Phase 3 Epic Trial of Iclusig in Patients with Newly Diagnosed Chronic Myeloid Leukemia". Archived from the original on 2013-10-18. Retrieved 2021-08-11.
- ↑ "FDA Grants Ponatinib Full Approval for Rare Leukemias". Archived from the original on 2020-03-22. Retrieved 2021-08-11.
- ↑ "Ponatinib Pathway, Pharmacokinetics/Pharmacodynamics". PharmGKB.
- ↑ Zhou T, Commodore L, Huang WS, Wang Y, Thomas M, Keats J, Xu Q, Rivera VM, Shakespeare WC, Clackson T, Dalgarno DC, Zhu X (January 2011). "Structural mechanism of the Pan-BCR-ABL inhibitor ponatinib (AP24534): lessons for overcoming kinase inhibitor resistance". Chemical Biology & Drug Design. 77 (1): 1–11. doi:10.1111/j.1747-0285.2010.01054.x. PMID 21118377. S2CID 22604788.
- ↑ "FDA asks manufacturer of the leukemia drug Iclusig (ponatinib) to suspend marketing and sales". FDA Drug Safety Communication. U.S. Food and Drug Administration. 2013-10-31. Archived from the original on 2017-11-02. Retrieved 2021-08-11.
- ↑ Grady D (2013-10-31). "Serious Danger of Blood Clots Halts Sale of Leukemia Drug". Business. New York Times. Archived from the original on 2021-09-18. Retrieved 2021-08-11.
- ↑ 15.0 15.1 Pollack A (April 25, 2013). "Doctors Denounce Cancer Drug Prices of $100,000 a Year". New York Times. Archived from the original on February 21, 2017. Retrieved August 11, 2021.
- ↑ 16.0 16.1 Experts in Chronic Myeloid Leukemia (May 2013). "The price of drugs for chronic myeloid leukemia (CML) is a reflection of the unsustainable prices of cancer drugs: from the perspective of a large group of CML experts". Blood. 121 (22): 4439–42. doi:10.1182/blood-2013-03-490003. PMC 4190613. PMID 23620577.
- ↑ NHS England. "National Cancer Drugs Fund list Ver4.3". Jun 5, 2015 (last retrieved Jul 20, 2015)
- ↑ National Institute for Health and Care Excellence. "Consultation on Batch 33 draft remits and draft scopes and summary of comments and discussions at scoping workshops" Archived 2018-11-01 at the Wayback Machine. 2014 (last retrieved Jul 20, 2015)
- ↑ Gever J (Dec 10, 2012). "Ponatinib Retains Luster in Leukemia". Oncology/Hematology. MedPageToday.com. Archived from the original on September 18, 2021. Retrieved August 11, 2021.
- ↑ "Ponatinib in Newly Diagnosed Chronic Myeloid Leukemia". 5 November 2014. Archived from the original on 19 October 2013. Retrieved 11 August 2021.
{{cite journal}}: Cite journal requires|journal=(help)
External links
| External sites: | |
|---|---|
| Identifiers: |
|
- Pages using duplicate arguments in template calls
- Webarchive template wayback links
- CS1 errors: missing periodical
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without UNII source
- Drugs missing an ATC code
- Drug has EMA link
- Articles containing unverified chemical infoboxes
- All articles with unsourced statements
- Articles with unsourced statements from December 2012
- Articles with invalid date parameter in template
- Articles with hatnote templates targeting a nonexistent page
- Wikipedia articles needing clarification from January 2016
- Alkyne derivatives
- Non-receptor tyrosine kinase inhibitors
- Trifluoromethyl compounds
- Piperazines
- Takeda Pharmaceutical Company brands
- RTT