Tocilizumab
| Monoclonal antibody | |
|---|---|
| Type | Whole antibody |
| Source | Humanized (from mouse) |
| Target | IL-6 receptor |
| Names | |
| Trade names | Actemra, RoActemra |
| Clinical data | |
| Drug class | Disease-modifying antirheumatic drug (biologic)[1] |
| Main uses | Rheumatoid arthritis (RA), juvenile idiopathic arthritis, COVID-19, giant cell arteritis[1][2] |
| Side effects | Upper respiratory tract infections, headache, high blood pressure[1] |
| Pregnancy category |
|
| Routes of use | Intravenous infusion, subcutaneous injection |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a611004 |
| Legal | |
| License data |
|
| Legal status | |
| Pharmacokinetics | |
| Elimination half-life | 8–14 days during steady state (dependent on concentration) |
| Chemical and physical data | |
| Formula | C6428H9976N1720O2018S42 |
| Molar mass | 144987.06 g·mol−1 |
Tocilizumab, also known as atlizumab and sold under the trade name Actemra among others is an immunosuppressive medication, used for rheumatoid arthritis (RA), juvenile idiopathic arthritis, COVID-19, and giant cell arteritis.[1][2] In RA it may be used when other treatments are not sufficiently effective.[1] In COVID-19 it is used in severe disease.[1][3] It is used by injection.[1]
Common side effects include upper respiratory tract infections, headache, and high blood pressure.[1] Other severe side effects include gastrointestinal perforation, low blood neutrophils, liver inflammation, anaphylaxis, and cancer.[1] Infection that occur as a result of tocilizumab may be severe.[1] Use in pregnancy and breastfeeding is not recommended.[2] It is a humanized monoclonal antibody against the interleukin-6 receptor (IL-6R).[1]
Tocilizumab was approved for medical use in Japan in 2005, Europe in 2009, and the United States in 2010.[1][4] It was developed by Chugai and Hoffmann–La Roche.[5] The form that is injected just under the skin is generally given every one to two weeks and costs the NHS 230 pounds per dose as of 2020.[2] In the United States this amount is 1,150 USD as of 2020.[6] Generic versions are not avaliable as of 2016 but are in development.[4]
Medical uses
The drug is administered by monthly intravenous infusions. An infusion takes about an hour.[7] An alternative formulation for subcutaneous injection was approved in October 2013.[8]
Rheumatoid arthritis
Tocilizumab is used for the treatment of moderate to severe rheumatoid arthritis, applied in combination with methotrexate, if other drugs like disease-modifying antirheumatic drugs (DMARDs) and TNF alpha blockers have proven to be ineffective or were not tolerated. It can be used by itself in people who do not tolerate methotrexate.[9] The drug slows down the progression of the disease and can improve physical function.[10]
Juvenile idiopathic arthritis
The treatment of systematic juvenile idiopathic arthritis is similar to RA treatment: tocilizumab is combined with methotrexate unless the latter is not tolerated. General safety and effectiveness is established for children of two years and older.[11]
In 2011 the US FDA approved tocilizumab for the treatment of active systemic juvenile idiopathic arthritis (SJIA).[12]
Giant cell arteritis
In May 2017, tocilizumab was FDA approved for giant cell arteritis (temporal arteritis).[13]
COVID-19
China's National Health Commission included the use of tocilizumab in guidelines to treat coronavirus disease 2019 (COVID-19) patients.[14] In March 2020, China approved tocilizumab for the treatment of inflammation in patients with the coronavirus SARS-CoV-2. As of June 2020[update], there is no evidence whether this treatment is effective.[15] Chinese health officials say that only 21 patients have been asked to use this medicine.[16] The same month, a randomized study, at 11 locations in China was conducted to compare favipiravir versus tocilizumab versus both.[17]
Roche announced in July 2020 that its randomized double-blind trial of tocilizumab in the treatment of pneumonia in Covid patients had shown no benefits of the drug.[18] In October 2020, another research was published that concluded that "tocilizumab was not effective for preventing intubation or death in moderately ill hospitalized patients with Covid-19".[19]
Other
In Japan, tocilizumab is also approved for the treatment of Castleman's disease,[20] a rare benign tumor of B cells.
There are case reports of use in otherwise refractory neuromyelitis optica.[21]
On 30 August 2017, the FDA approved tocilizumab for cytokine release syndrome, a side effect of CAR-T cell therapies.[22]
Dosage
The typical starting dose for RA in those who weight less than 100 kg is 162 mg injected just under the skin every two weeks which may be increased to weekly.[1]
Side effects
The most common side effects are upper respiratory tract infections (more than 10% of patients), nasopharyngitis (common cold), headache, and high blood pressure (at least 5%). The enzyme alanine transaminase was also elevated in at least 5% of people, but in most cases without symptoms. Elevated total cholesterol levels were common.[23] Among the less common side effects were dizziness, various infections, as well as reactions of the skin and mucosae like mild rashes, gastritis and mouth ulcer. Rare but severe reactions were gastrointestinal perforations (0.26% in six months) and anaphylaxis (0.2%).[24]
The application of tocilizumab is contraindicated during acute infections, as well as under latent tuberculosis.[24]
Pregnancy and breastfeeding
No clinical studies evaluating the risk for the baby. A study using large doses of tocilizumab in pregnant animals has found an increased likelihood for spontaneous abortion. It is not known whether the drug is secreted into the breast milk, nor if this would pose a risk for the nursling.[9]
Interactions
There are no certain interactions with other drugs. The blood plasma levels of simvastatin were reduced by 57% after a single dose of tocilizumab, but it is not known whether this is clinically relevant. A possible mechanism is that the elevated IL-6 levels of patients with RA suppress the biosynthesis of various cytochrome P450 enzymes, notably CYP1A2, CYP2C9, CYP2C19 and CYP3A4. Tocilizumab lowers IL-6 and thus normalises cytochrome levels, increasing the metabolization of simvastatin (and possibly other cytochrome metabolised drugs).[24]
Mechanism of action
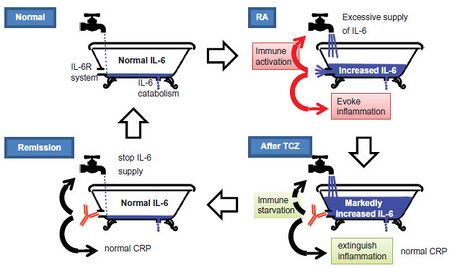
Besides other functions, interleukin 6 (IL-6) is involved in the development of immunological and inflammatory reactions. Some autoimmune diseases like RA are associated with abnormally high IL-6 levels. Tocilizumab binds soluble as well as membrane bound interleukin-6 receptors, hindering IL-6 from exerting its pro-inflammatory effects.[24][26] It has been noted that the membrane bound form and soluble form of the IL-6 receptor may have different effects in the pathogenesis of rheumatoid arthritis with the soluble form being more implicated in disease progression.[27]
History
Interleukin 6 and its receptor were discovered and cloned at Osaka University, Japan, by Tadamitsu Kishimoto in the 1980s. In 1997, Chugai Pharmaceuticals began the clinical development of tocilizumab for the treatment of rheumatoid arthritis. Clinical studies for Castleman's disease and systemic juvenile idiopathic arthritis started in 2001 and 2002, respectively. Hoffmann–La Roche co-developed the drug due to a license agreement in 2003.[28]
Data presented in 2008 showed the effectiveness of tocilizumab in combination therapy with methotrexate for RA treatment.[29] In further studies, it was effective and generally well tolerated when administered either as monotherapy or in combination with conventional DMARDs in adult patients with moderate to severe rheumatoid arthritis.[30]
In June 2005, tocilizumab was approved in Japan for Castleman's disease. In January 2009, the drug was approved by the European Medicines Agency (EMA) as RoActemra for the treatment of rheumatoid arthritis under the mentioned restrictions. On 11 January 2010, it was approved by the U.S. Food and Drug Administration (US FDA) as Actemra for the same purpose.[31] Tocilizumab was approved by Australia's Therapeutic Goods Administration on 27 May 2009[32] and was listed on the Pharmaceutical Benefits Scheme from 1 August 2010.[33] In New Zealand, tocilizumab was approved for distribution in July 2009,[34] and Pharmac approved subsidising it with special authority restrictions on 1 July 2013 for systemic juvenile idiopathic arthritis[35] and 1 July 2014 for rheumatoid arthritis.[36] The FDA approved tocilizumab for the treatment of systemic juvenile idiopathic arthritis for children from two years of age in April 2011, and the EMA followed in August the same year.
Tocilizumab is marketed by Chugai in some countries, especially in Japan and other Asian countries, and jointly by Chugai and Roche (Hoffmann–La Roche's holding company) in others, for example Great Britain, France and Germany.[28]
Research
The intensity of IL-6 in cancer cells from people with ovarian cancer is associated with poor prognosis, and in cell culture a different anti-IL-6 antibody (siltuximab) reduced IL-6 signaling and showed favorable effects in an animal model of the disease; however, the drug showed no clear effects when tested in a phase II clinical trial in people with advanced ovarian cancer.[37] The trial investigators later showed that in high-grade ovarian cancer cells, anti-IL6 antibodies reduced the activation of STAT3 (its major downstream transcription factor), leading to increased activity of the epidermal growth factor receptor (EGFR) pathway, which is thought to confer resistance to several cancer therapies.[38] The investigators then showed that inhibiting this pathway with the EGFR inhibitor gefitinib inhibited tumor growth was in cell culture and animals of the disease, offering a potential rationale for combining anti-IL6 antibodies with gefitinib to treat advanced-stage ovarian cancer.[38]
Tocilizumab is being studied for pulmonary arterial hypertension (PAH).[39] Tocilizumab is currently under evaluation in a multicenter clinical trial (ALL-IN) for the prevention of acute cellular rejection in status post heart transplant patients.[40]
See also
- Anti-IL-6 medications
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 "Tocilizumab Monograph for Professionals". Drugs.com. Archived from the original on 14 October 2020. Retrieved 16 October 2020.
- ↑ 2.0 2.1 2.2 2.3 BNF 79. London: Pharmaceutical Press. March 2020. p. 1137. ISBN 978-0857113658.
- ↑ "Hospitalized Adults: Therapeutic Management". COVID-19 Treatment Guidelines. Archived from the original on 9 January 2022. Retrieved 8 January 2022.
- ↑ 4.0 4.1 "Biosimilars of tocilizumab / General / Biosimilars / Home - GaBI Online - Generics and Biosimilars Initiative". gabionline.net. Archived from the original on 6 August 2020. Retrieved 16 October 2020.
- ↑ Venkiteshwaran, Adith (2009). "Tocilizumab". mAbs. 1 (5): 432–438. doi:10.4161/mabs.1.5.9497. PMC 2759492. PMID 20065633. Archived from the original on 8 March 2012.
- ↑ "Tocilizumab Prices, Coupons & Savings Tips". GoodRx. Archived from the original on 29 August 2021. Retrieved 16 October 2020.
- ↑ Haberfeld, H, ed. (2009). Austria-Codex (in German) (2009/2010 ed.). Vienna: Österreichischer Apothekerverlag. ISBN 978-3-85200-196-8.
{{cite book}}: CS1 maint: unrecognized language (link) - ↑ "Genentech Gains FDA Approval for New Subcutaneous Formulation of Actemra for use in Adult Patients Living with Moderately to Severely Active Rheumatoid Arthritis". Genentech. 21 October 2013. Archived from the original on 8 September 2017.
- ↑ 9.0 9.1 "Assessment report for RoActemra" (PDF). European Medicines Agency. Archived (PDF) from the original on 2016-01-19. Retrieved 2011-10-06.
- ↑ Roy Fleischmann; et al. (2009). "LITHE: Tocilizumab Inhibits Radiographic Progression and Improves Physical Function in Rheumatoid Arthritis (RA) Patients (Pts) at 2 Yrs with Increasing Clinical Efficacy Over Time". ACR. Archived from the original on 2020-08-09. Retrieved 2020-08-01.
{{cite journal}}: Cite journal requires|journal=(help) - ↑ Chaitow, J; De Benedetti, F; Brunner, H; et al. (2010). "Tocilizumab in Patients With Systemic Juvenile Idiopathic Arthritis: Efficacy Data From the Placebo-Controlled 12-Week Part of the Phase 3 TENDER Trial" (PDF). Arthritis & Rheumatism. 62 (Supplement 10): 1434. Archived (PDF) from the original on 2019-12-31. Retrieved 2020-08-01.
- ↑ FDA Approves ACTEMRA® (tocilizumab) for the Treatment of Systemic Juvenile Idiopathic Arthritis (SJIA), South San Francisco, California: Genentech, April 15, 2011, archived from the original on 1 October 2018, retrieved July 20, 2015
- ↑ "FDA Approves Actemra (tocilizumab) Subcutaneous Injection for Giant Cell Arteritis". Drugs.com. Archived from the original on 2020-08-09. Retrieved 2017-05-25.
- ↑ "China approves use of Roche drug in battle against coronavirus complications". March 4, 2020. Archived from the original on March 5, 2020. Retrieved March 31, 2020 – via www.reuters.com.
- ↑ "China approves use of Roche arthritis drug for coronavirus patients". Reuters. 2020-03-04. Archived from the original on 2020-03-12. Retrieved 2020-03-04.
- ↑ "Coronavirus, farmaco per curare l'artrite reumatoide efficace su due pazienti con polmonite severa da Covid 19" [Coronavirus, drug to treat rheumatoid arthritis effective on two patients with severe pneumonia from Covid 19] (in Italian). Il fatto quotidiano. 2020-03-09. Archived from the original on 2020-08-09. Retrieved 2020-03-10.
{{cite news}}: CS1 maint: unrecognized language (link) - ↑ "Favipiravir Combined With Tocilizumab in the Treatment of Corona Virus Disease 2019". National Library of Medicine. March 17, 2020. Archived from the original on February 25, 2021. Retrieved April 9, 2020.
- ↑ "Roche provides an update on the phase III COVACTA trial of Actemra/RoActemra in hospitalised patients with severe COVID-19 associated pneumonia". www.roche.com. 29 July 2020. Archived from the original on 2021-01-09. Retrieved 2020-08-18.
- ↑ Stone, John H.; Frigault, Matthew J.; Serling-Boyd, Naomi J.; Fernandes, Ana D.; Harvey, Liam; Foulkes, Andrea S.; Horick, Nora K.; Healy, Brian C.; Shah, Ruta; Bensaci, Ana Maria; Woolley, Ann E. (2020-10-21). "Efficacy of Tocilizumab in Patients Hospitalized with Covid-19". New England Journal of Medicine. 0 (0): null. doi:10.1056/NEJMoa2028836. ISSN 0028-4793. Archived from the original on 2021-08-29. Retrieved 2020-10-23.
- ↑ Matsuyama M; Suzuki T; Tsuboi H; et al. (2007). "Anti-interleukin-6 receptor antibody (tocilizumab) treatment of multicentric Castleman's disease". Intern. Med. 46 (11): 771–4. doi:10.2169/internalmedicine.46.6262. PMID 17541233.
- ↑ Lin, J; Xue, B; Li, X; Xia, J (August 2017). "Monoclonal antibody therapy for neuromyelitis optica spectrum disorder: current and future". The International journal of neuroscience. 127 (8): 735–744. doi:10.1080/00207454.2016.1242587. PMID 27680606.
- ↑ "FDA approves tisagenlecleucel for B-cell ALL and tocilizumab for cytokine release syndrome". US FDA. 30 August 2017. Archived from the original on 29 August 2021. Retrieved 5 September 2017.
- ↑ Genovese, M. C.; McKay, J. D.; Nasonov, E. L.; Mysler, E. F.; Da Silva, N. A.; Alecock, E.; Woodworth, T.; Gomez-Reino, J. J. (2008). "Interleukin-6 receptor inhibition with tocilizumab reduces disease activity in rheumatoid arthritis with inadequate response to disease-modifying antirheumatic drugs: The tocilizumab in combination with traditional disease-modifying antirheumatic drug therapy study". Arthritis & Rheumatism. 58 (10): 2968–80. doi:10.1002/art.23940. PMID 18821691.
- ↑ 24.0 24.1 24.2 24.3 Dinnendahl, V; Fricke, U, eds. (2010). Arzneistoff-Profile (in German). Vol. 4 (23 ed.). Eschborn, Germany: Govi Pharmazeutischer Verlag. ISBN 978-3-7741-9846-3.
{{cite book}}: CS1 maint: unrecognized language (link) - ↑ Ogata, Atsushi; Hirano, Toru; Hishitani, Yoshihiro; Tanaka, Toshio (January 2012). "Safety and Efficacy of Tocilizumab for the Treatment of Rheumatoid Arthritis". Clinical Medicine Insights: Arthritis and Musculoskeletal Disorders. 5: CMAMD.S7371. doi:10.4137/CMAMD.S7371. ISSN 1179-5441.
- ↑ Jones, G; Sebba, A; Gu, J; Lowenstein, MB; Calvo, A; Gomez-Reino, JJ; Siri, DA; Tomsic, M; et al. (2010). "Comparison of tocilizumab monotherapy versus methotrexate monotherapy in patients with moderate to severe rheumatoid arthritis: The AMBITION study". Annals of the Rheumatic Diseases. 69 (1): 88–96. doi:10.1136/ard.2008.105197. PMC 3747519. PMID 19297346.
- ↑ Kallen, K.J. (2002). "The role of transsignalling via the agonistic soluble IL-6 receptor in human diseases". Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 1592 (3): 323–343. doi:10.1016/s0167-4889(02)00325-7. PMID 12421676.
- ↑ 28.0 28.1 Markus Harwart (2008). "Die Entwicklung von Tocilizumab" [The development of tocilizumab] (in German). Krankenpflege-Journal. Archived from the original on 15 October 2018.
{{cite web}}: CS1 maint: unrecognized language (link) - ↑ "Jab hope for rheumatoid arthritis". October 27, 2008. Archived from the original on January 26, 2021. Retrieved October 27, 2008 – via news.bbc.co.uk.
- ↑ Oldfield, V; Dhillon, S; Plosker, GL (2009). "Tocilizumab". Drugs. 69 (5): 609–632. doi:10.2165/00003495-200969050-00007. PMID 19368420. Archived from the original on 2013-01-16. Retrieved 2010-03-17.
- ↑ "Roche: FDA Approves Actemra For Rheumatoid Arthritis". The Wall Street Journal. 11 January 2010. Archived from the original on January 14, 2010.
- ↑ "Australian Drug Evaluation Committee 263rd meeting resolutions". Therapeutic Goods Administration. 27 May 2009. Archived from the original on 20 August 2009.
- ↑ "Anakinra (Kineret) to be deleted from the PBS". National Prescribing Service Limited. 1 August 2010. Archived from the original on 3 June 2012.
- ↑ Richards, Mark (20 July 2009). "Consent to the Distribution of New Medicines". New Zealand Gazette. 2009 (105): 2418. Archived from the original on 9 August 2020. Retrieved 9 June 2015.
- ↑ "Approval of proposal involving pegfilgrastim and tocilizumab" (PDF). Pharmaceutical Management Agency. 24 May 2013. Retrieved 9 June 2015.
- ↑ "Decision to widen access to tocilizumab (Actemra) for rheumatoid arthritis in patients who are unable to be treated with methotrexate" (PDF). Pharmaceutical Management Agency. 14 May 2014. Retrieved 9 June 2015.
- ↑ Coward J, Kulbe H, Chakravarty P, Leader D, Vassileva V, Leinster DA, Thompson R, Schioppa T, Nemeth J, Vermeulen J, Singh N, Avril N, Cummings J, Rexhepaj E, Jirström K, Gallagher WM, Brennan DJ, McNeish IA, Balkwill FR (September 15, 2011). "Interleukin-6 as a therapeutic target in human ovarian cancer". Clin Cancer Res. 17 (18): 6083–96. doi:10.1158/1078-0432.CCR-11-0945. PMC 3182554. PMID 21795409.
- ↑ 38.0 38.1 Milagre CS, Gopinathan G, Everitt G, Thompson RG, Kulbe H, Zhong H, Hollingsworth RE, Grose R, Bowtell DD, Hochhauser D, Balkwill FR (April 1, 2015). "Adaptive upregulation of EGFR limits attenuation of tumor growth by neutralizing IL6 antibodies, with implications for combined therapy in ovarian cancer". Cancer Res. 75 (7): 1255–64. doi:10.1158/0008-5472.CAN-14-1801. PMC 4384986. PMID 25670170.
- ↑ "Roche links with UK gov for ground-breaking PAH trial". PharmaTimes Media Ltd. 2016-01-06. Archived from the original on 2016-03-06. Retrieved 2016-01-19.
- ↑ Clinical trial number NCT03644667 for "Tocilizumab in Cardiac Transplantation" at ClinicalTrials.gov
External links
| External sites: | |
|---|---|
| Identifiers: |
|
- Pages using duplicate arguments in template calls
- CS1 maint: unrecognized language
- CS1 errors: missing periodical
- Drugs with non-standard legal status
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Drugs missing an ATC code
- Drug has EMA link
- Drugboxes which contain changes to verified fields
- Drugboxes which contain changes to watched fields
- Drugs that are a monoclonal antibody
- Articles containing potentially dated statements from June 2020
- Articles with invalid date parameter in template
- All articles containing potentially dated statements
- Articles with changed CASNo identifier
- Chemicals that do not have a ChemSpider ID assigned
- Articles with changed EBI identifier
- Immunosuppressants
- Orphan drugs
- Hoffmann-La Roche brands
- Genentech brands
- RTT
- RTTCovid