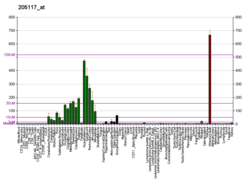
FGF1
Fibroblast growth factor 1, (FGF-1) also known as acidic fibroblast growth factor (aFGF), is a growth factor and signaling protein encoded by the FGF1 gene.[5][6] It is synthesized as a 155 amino acid polypeptide, whose mature form is a non-glycosylated 17-18 kDa protein. Fibroblast growth factor protein was first purified in 1975, but soon afterwards others using different conditions isolated acidic FGF, Heparin-binding growth factor-1, and Endothelial cell growth factor-1.[7] Gene sequencing revealed that this group was actually the same growth factor and that FGF1 was a member of a family of FGF proteins.
FGF-1 has no definitive signal sequence and thus is not secreted through classical pathways, but it does appear to form a disulfide linked dimer inside cells that associate with a complex of proteins at the cell membrane (including S100A13 and Syt1) which then help flip it through the membrane to the exterior of the cell.[8][9] Once in the reducing conditions of the surrounding tissue, the dimer dissociates into monomeric FGF1 that can enter systemic circulation or be sequestered in tissues binding to heparan sulfate proteoglycans of the extracellular matrix. FGF1 can then bind to and exert its effects via specific fibroblast growth factor receptor (FGFR) proteins which themselves constitute a family of closely related molecules.[10]
In addition to its extracellular activity, FGF1 can also function intracellularly. The protein has a nuclear localization sequence (NLS) but the route that FGF1 takes to get to the nucleus is unclear and it appears that some sort of cell surface receptor binding is necessary, followed by its internalization and translocation to the nucleus whereupon it can interact with nuclear isoforms of FGFRs.[10] This is different from FGF2 which also can activate nuclear FGFRs but has splicing variants of the protein that never leave the cell and go directly to the nucleus.[citation needed]
Function
FGF family members possess broad mitogenic and cell survival activities, and are involved in a variety of biological processes, including embryonic development, cell growth, morphogenesis, tissue repair, tumor growth and invasion. This protein functions as a modifier of endothelial cell migration and proliferation, as well as an angiogenic factor. It acts as a mitogen for a variety of mesoderm- and neuroectoderm-derived cells in vitro, thus is thought to be involved in organogenesis. Three alternatively spliced variants encoding different isoforms have been described.[11]
FGF1 is multifunctional with many reported effects. For one example, in mice with diet-induced diabetes that is an experimental equivalent of type 2 diabetes in humans, a single injection of the FGF1 protein is enough to restore blood sugar levels to a healthy range for > 2 days.[12]
Interactions
FGF1 has been shown to interact with:
See also
References
- ^ a b c GRCh38: Ensembl release 89: ENSG00000113578 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000036585 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Dionne CA, Crumley G, Bellot F, Kaplow JM, Searfoss G, Ruta M, Burgess WH, Jaye M, Schlessinger J (September 1990). "Cloning and expression of two distinct high-affinity receptors cross-reacting with acidic and basic fibroblast growth factors". The EMBO Journal. 9 (9): 2685–92. doi:10.1002/j.1460-2075.1990.tb07454.x. PMC 551973. PMID 1697263.
- ^ Jaye M, Howk R, Burgess W, Ricca GA, Chiu IM, Ravera MW, O'Brien SJ, Modi WS, Maciag T, Drohan WN (August 1986). "Human endothelial cell growth factor: cloning, nucleotide sequence, and chromosome localization". Science. 233 (4763): 541–5. Bibcode:1986Sci...233..541J. doi:10.1126/science.3523756. PMID 3523756.
- ^ Burgess WH, Maciag T (1989). "The heparin-binding (fibroblast) growth factor family of proteins". Annual Review of Biochemistry. 58: 575–606. doi:10.1146/annurev.bi.58.070189.003043. PMID 2549857.
- ^ Tarantini F, Gamble S, Jackson A, Maciag T (December 1995). "The cysteine residue responsible for the release of fibroblast growth factor-1 residues in a domain independent of the domain for phosphatidylserine binding". The Journal of Biological Chemistry. 270 (49): 29039–42. doi:10.1074/jbc.270.49.29039. PMID 7493920.
- ^ a b c Prudovsky I, Bagala C, Tarantini F, Mandinova A, Soldi R, Bellum S, Maciag T (July 2002). "The intracellular translocation of the components of the fibroblast growth factor 1 release complex precedes their assembly prior to export". The Journal of Cell Biology. 158 (2): 201–8. doi:10.1083/jcb.200203084. PMC 2173119. PMID 12135982.
- ^ a b Coleman SJ, Bruce C, Chioni AM, Kocher HM, Grose RP (August 2014). "The ins and outs of fibroblast growth factor receptor signalling". Clinical Science. 127 (4): 217–31. doi:10.1042/CS20140100. PMID 24780002.
- ^ "Entrez Gene: FGF1 fibroblast growth factor 1 (acidic)".
- ^ Suh JM, Jonker JW, Ahmadian M, Goetz R, Lackey D, Osborn O, Huang Z, Liu W, Yoshihara E, van Dijk TH, Havinga R, Fan W, Yin YQ, Yu RT, Liddle C, Atkins AR, Olefsky JM, Mohammadi M, Downes M, Evans RM (September 2014). "Endocrinization of FGF1 produces a neomorphic and potent insulin sensitizer". Nature. 513 (7518): 436–9. Bibcode:2014Natur.513..436S. doi:10.1038/nature13540. PMC 4184286. PMID 25043058.*Lay summary in: "One injection stops diabetes in its tracks". Salk Institute. July 16, 2014.
- ^ a b c Skjerpen CS, Nilsen T, Wesche J, Olsnes S (August 2002). "Binding of FGF-1 variants to protein kinase CK2 correlates with mitogenicity". The EMBO Journal. 21 (15): 4058–69. doi:10.1093/emboj/cdf402. PMC 126148. PMID 12145206.
- ^ Kolpakova E, Wiedłocha A, Stenmark H, Klingenberg O, Falnes PO, Olsnes S (November 1998). "Cloning of an intracellular protein that binds selectively to mitogenic acidic fibroblast growth factor". The Biochemical Journal. 336 (1): 213–22. doi:10.1042/bj3360213. PMC 1219860. PMID 9806903.
- ^ Schlessinger J, Plotnikov AN, Ibrahimi OA, Eliseenkova AV, Yeh BK, Yayon A, Linhardt RJ, Mohammadi M (September 2000). "Crystal structure of a ternary FGF-FGFR-heparin complex reveals a dual role for heparin in FGFR binding and dimerization". Molecular Cell. 6 (3): 743–50. doi:10.1016/s1097-2765(00)00073-3. PMID 11030354.
- ^ a b c Santos-Ocampo S, Colvin JS, Chellaiah A, Ornitz DM (January 1996). "Expression and biological activity of mouse fibroblast growth factor-9". The Journal of Biological Chemistry. 271 (3): 1726–31. doi:10.1074/jbc.271.3.1726. PMID 8576175.
- ^ Stauber DJ, DiGabriele AD, Hendrickson WA (January 2000). "Structural interactions of fibroblast growth factor receptor with its ligands". Proceedings of the National Academy of Sciences of the United States of America. 97 (1): 49–54. Bibcode:2000PNAS...97...49S. doi:10.1073/pnas.97.1.49. PMC 26614. PMID 10618369.
- ^ Pellegrini L, Burke DF, von Delft F, Mulloy B, Blundell TL (October 2000). "Crystal structure of fibroblast growth factor receptor ectodomain bound to ligand and heparin". Nature. 407 (6807): 1029–34. Bibcode:2000Natur.407.1029P. doi:10.1038/35039551. PMID 11069186. S2CID 4418272.
- ^ Chellaiah A, Yuan W, Chellaiah M, Ornitz DM (December 1999). "Mapping ligand binding domains in chimeric fibroblast growth factor receptor molecules. Multiple regions determine ligand binding specificity". The Journal of Biological Chemistry. 274 (49): 34785–94. doi:10.1074/jbc.274.49.34785. PMID 10574949.
- ^ Loo BB, Darwish KK, Vainikka SS, Saarikettu JJ, Vihko PP, Hermonen JJ, Goldman AA, Alitalo KK, Jalkanen MM (May 2000). "Production and characterization of the extracellular domain of recombinant human fibroblast growth factor receptor 4". The International Journal of Biochemistry & Cell Biology. 32 (5): 489–97. doi:10.1016/S1357-2725(99)00145-4. PMID 10736564.
- ^ Kan M, Wu X, Wang F, McKeehan WL (May 1999). "Specificity for fibroblast growth factors determined by heparan sulfate in a binary complex with the receptor kinase". The Journal of Biological Chemistry. 274 (22): 15947–52. doi:10.1074/jbc.274.22.15947. PMID 10336501.
- ^ Mizukoshi E, Suzuki M, Loupatov A, Uruno T, Hayashi H, Misono T, Kaul SC, Wadhwa R, Imamura T (October 1999). "Fibroblast growth factor-1 interacts with the glucose-regulated protein GRP75/mortalin". The Biochemical Journal. 343 (2): 461–6. doi:10.1042/0264-6021:3430461. PMC 1220575. PMID 10510314.
- ^ a b Mouta Carreira C, LaVallee TM, Tarantini F, Jackson A, Lathrop JT, Hampton B, Burgess WH, Maciag T (August 1998). "S100A13 is involved in the regulation of fibroblast growth factor-1 and p40 synaptotagmin-1 release in vitro". The Journal of Biological Chemistry. 273 (35): 22224–31. doi:10.1074/jbc.273.35.22224. hdl:2158/26736. PMID 9712836.
- ^ Landriscina M, Bagalá C, Mandinova A, Soldi R, Micucci I, Bellum S, Prudovsky I, Maciag T (July 2001). "Copper induces the assembly of a multiprotein aggregate implicated in the release of fibroblast growth factor 1 in response to stress". The Journal of Biological Chemistry. 276 (27): 25549–57. doi:10.1074/jbc.M102925200. PMID 11432880.
Further reading
- Yu YL, Kha H, Golden JA, Migchielsen AA, Goetzl EJ, Turck CW (April 1992). "An acidic fibroblast growth factor protein generated by alternate splicing acts like an antagonist". The Journal of Experimental Medicine. 175 (4): 1073–80. doi:10.1084/jem.175.4.1073. PMC 2119192. PMID 1372643.
- Chiu IM, Wang WP, Lehtoma K (May 1990). "Alternative splicing generates two forms of mRNA coding for human heparin-binding growth factor 1". Oncogene. 5 (5): 755–62. PMID 1693186.
- Zhu X, Komiya H, Chirino A, Faham S, Fox GM, Arakawa T, Hsu BT, Rees DC (January 1991). "Three-dimensional structures of acidic and basic fibroblast growth factors". Science. 251 (4989): 90–3. Bibcode:1991Sci...251...90Z. doi:10.1126/science.1702556. PMID 1702556.
- Wang WP, Quick D, Balcerzak SP, Needleman SW, Chiu IM (September 1991). "Cloning and sequence analysis of the human acidic fibroblast growth factor gene and its preservation in leukemia patients". Oncogene. 6 (9): 1521–9. PMID 1717925.
- Wu DQ, Kan MK, Sato GH, Okamoto T, Sato JD (September 1991). "Characterization and molecular cloning of a putative binding protein for heparin-binding growth factors". The Journal of Biological Chemistry. 266 (25): 16778–85. doi:10.1016/S0021-9258(18)55368-0. PMID 1885605.
- Crumley G, Dionne CA, Jaye M (August 1990). "The gene for human acidic fibroblast growth factor encodes two upstream exons alternatively spliced to the first coding exon". Biochemical and Biophysical Research Communications. 171 (1): 7–13. doi:10.1016/0006-291X(90)91348-V. PMID 2393407.
- Harper JW, Strydom DJ, Lobb RR (July 1986). "Human class 1 heparin-binding growth factor: structure and homology to bovine acidic brain fibroblast growth factor". Biochemistry. 25 (14): 4097–103. doi:10.1021/bi00362a017. PMID 2427112.
- Winkles JA, Friesel R, Burgess WH, Howk R, Mehlman T, Weinstein R, Maciag T (October 1987). "Human vascular smooth muscle cells both express and respond to heparin-binding growth factor I (endothelial cell growth factor)". Proceedings of the National Academy of Sciences of the United States of America. 84 (20): 7124–8. Bibcode:1987PNAS...84.7124W. doi:10.1073/pnas.84.20.7124. PMC 299242. PMID 2444975.
- Wang WP, Lehtoma K, Varban ML, Krishnan I, Chiu IM (June 1989). "Cloning of the gene coding for human class 1 heparin-binding growth factor and its expression in fetal tissues". Molecular and Cellular Biology. 9 (6): 2387–95. doi:10.1128/mcb.9.6.2387. PMC 362312. PMID 2474753.
- Mergia A, Tischer E, Graves D, Tumolo A, Miller J, Gospodarowicz D, Abraham JA, Shipley GD, Fiddes JC (November 1989). "Structural analysis of the gene for human acidic fibroblast growth factor". Biochemical and Biophysical Research Communications. 164 (3): 1121–9. doi:10.1016/0006-291X(89)91785-3. PMID 2590193.
- Gimenez-Gallego G, Conn G, Hatcher VB, Thomas KA (July 1986). "The complete amino acid sequence of human brain-derived acidic fibroblast growth factor". Biochemical and Biophysical Research Communications. 138 (2): 611–7. doi:10.1016/S0006-291X(86)80540-X. PMID 3527167.
- Gautschi P, Fràter-Schröder M, Böhlen P (August 1986). "Partial molecular characterization of endothelial cell mitogens from human brain: acidic and basic fibroblast growth factors". FEBS Letters. 204 (2): 203–7. doi:10.1016/0014-5793(86)80812-2. PMID 3732516. S2CID 22617694.
- Gautschi-Sova P, Müller T, Böhlen P (November 1986). "Amino acid sequence of human acidic fibroblast growth factor". Biochemical and Biophysical Research Communications. 140 (3): 874–80. doi:10.1016/0006-291X(86)90716-3. PMID 3778488.
- Gimenez-Gallego G, Conn G, Hatcher VB, Thomas KA (March 1986). "Human brain-derived acidic and basic fibroblast growth factors: amino terminal sequences and specific mitogenic activities". Biochemical and Biophysical Research Communications. 135 (2): 541–8. doi:10.1016/0006-291X(86)90028-8. PMID 3964259.
- Zhao XM, Yeoh TK, Hiebert M, Frist WH, Miller GG (November 1993). "The expression of acidic fibroblast growth factor (heparin-binding growth factor-1) and cytokine genes in human cardiac allografts and T cells". Transplantation. 56 (5): 1177–82. doi:10.1097/00007890-199311000-00025. PMID 7504343.
- Pineda-Lucena A, Jiménez MA, Nieto JL, Santoro J, Rico M, Giménez-Gallego G (September 1994). "1H-NMR assignment and solution structure of human acidic fibroblast growth factor activated by inositol hexasulfate". Journal of Molecular Biology. 242 (1): 81–98. doi:10.1006/jmbi.1994.1558. PMID 7521397.
- Chotani MA, Payson RA, Winkles JA, Chiu IM (February 1995). "Human fibroblast growth factor 1 gene expression in vascular smooth muscle cells is modulated via an alternate promoter in response to serum and phorbol ester". Nucleic Acids Research. 23 (3): 434–41. doi:10.1093/nar/23.3.434. PMC 306694. PMID 7533902.
- Opalenik SR, Shin JT, Wehby JN, Mahesh VK, Thompson JA (July 1995). "The HIV-1 TAT protein induces the expression and extracellular appearance of acidic fibroblast growth factor". The Journal of Biological Chemistry. 270 (29): 17457–67. doi:10.1074/jbc.270.29.17457. PMID 7542239.
- Myers RL, Payson RA, Chotani MA, Deaven LL, Chiu IM (February 1993). "Gene structure and differential expression of acidic fibroblast growth factor mRNA: identification and distribution of four different transcripts". Oncogene. 8 (2): 341–9. PMID 7678925.