Romosozumab
| Monoclonal antibody | |
|---|---|
| Type | Whole antibody |
| Source | Humanized (from mouse) |
| Target | Sclerostin |
| Names | |
| Trade names | Evenity |
| Other names | AMG 785, romosozumab-aqqg |
| Clinical data | |
| Main uses | Osteoporosis[1] |
| Side effects | Headache, joint pain, allergic reaction[2] |
| Pregnancy category |
|
| Typical dose | 210 mg[1] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a619026 |
| Legal | |
| License data |
|
| Legal status | |
| Chemical and physical data | |
| Formula | C6452H9926N1714O2040S54 |
| Molar mass | 145877.58 g·mol−1 |
Romosozumab, sold under the brand name Evenity, is a medication used to treat osteoporosis.[1] It decreases the risk of fractures of the spine.[1] It is used by injection under the skin.[1]
Common side effect include headache, joint pain, and allergic reactions.[2] It may increase the risk of heart attacks and strokes.[3] Other side effects may include low calcium.[1] It is a monoclonal antibody that attaching to and blocks sclerostin.[1] This increases bone formation and reduces bone breakdown.[1]
Romosozumab was approved for medical use in the United States and Europe in 2019.[1][3] In the United States it costs about $2,050 a month as of 2021.[4] This amount in the United Kingdom costs the NHS about £430.[2]
Medical uses
Romosozumab is used for osteoporosis to decrease the risk of fractures.[5] Two trials found that it reduced the rate of vertebral fracture. In one, there was a 73% lower risk of vertebral fracture after one year, and the benefit was maintained after a second year of taking denosumab. In the other, one year of romosozumab followed by one year of alendronate had a 50% vertebral fracture reduction compared to two years of alendronate.[5]
Dosage
It is used at a dose of 210 mg once a month for up to a year.[1]
Side effects
Common side effects include headache, joint pain, and pain at the site of injection.[6] It may increase the risk of heart attacks, strokes, and deaths from cardiovascular disease.[6]
Mechanism of action
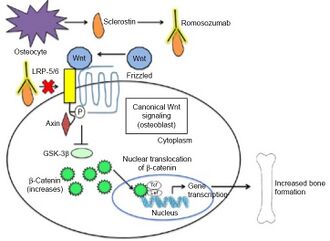
Romosozumab inhibits sclerostin from inhibiting bone formation which it usually does via binding to LDL receptor-related proteins 5/6 of osteoblasts. As a consequence, Romosozumab permits Wnt signaling in osteoblasts which promotes bone formation [7]
History
Romosozumab was approved for medical use in Japan in January 2019,[5] the United States in April 2019[5] and the European Union in December 2019.[8] It was originally discovered by Chiroscience,[9] which was acquired by Celltech (now[when?] owned by UCB).[10] Celltech entered in a partnership with Amgen in 2002 for the product's development.[11]
The U.S. Food and Drug Administration (FDA) considers it to be a first-in-class medication.[12]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 1.9 "Evenity". Archived from the original on 7 January 2021. Retrieved 18 October 2021.
- ↑ 2.0 2.1 2.2 BNF (80 ed.). BMJ Group and the Pharmaceutical Press. September 2020 – March 2021. p. 776. ISBN 978-0-85711-369-6.
- ↑ 3.0 3.1 "Romosozumab-aqqg Monograph for Professionals". Drugs.com. Retrieved 18 October 2021.
- ↑ "Evenity Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 19 April 2021. Retrieved 18 October 2021.
- ↑ 5.0 5.1 5.2 5.3 Kaplon H, Muralidharan M, Schneider Z, Reichert JM (2020). "Antibodies to watch in 2020". mAbs. 12 (1): 1703531. doi:10.1080/19420862.2019.1703531. PMC 6973335. PMID 31847708.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ 6.0 6.1 "FDA approves new treatment for osteoporosis in postmenopausal women at high risk of fracture". U.S. Food and Drug Administration (FDA) (Press release). 9 April 2019. Archived from the original on 2 May 2019. Retrieved 12 April 2019.
- ↑ 7.0 7.1 Lim, Sian Yik; Bolster, Marcy B. (13 April 2017). "Profile of romosozumab and its potential in the management of osteoporosis". Drug Design, Development and Therapy. 11: 1221–1231. doi:10.2147/DDDT.S127568. Archived from the original on 28 September 2022. Retrieved 13 February 2024.
- ↑ Victoria Rees (13 December 2019). "EC approves treatment for severe osteoporosis postmenopausal women". European Pharmaceutical Review. Archived from the original on 28 February 2020. Retrieved 27 February 2020.
- ↑ Quested T (7 June 2015). "Cream of life science entrepreneurs' first venture was selling doughnuts". Business Weekly. Cambridge, England: Q Communications. Archived from the original on 24 December 2018. Retrieved 24 December 2018.
- ↑ Winkler DG, Sutherland MK, Geoghegan JC, Yu C, Hayes T, Skonier JE, et al. (December 2003). "Osteocyte control of bone formation via sclerostin, a novel BMP antagonist". The EMBO Journal. 22 (23): 6267–6276. doi:10.1093/emboj/cdg599. PMC 291840. PMID 14633986.
- ↑ "Celltech group Interim Report 2002" (PDF). Celltech Group plc. Archived (PDF) from the original on 19 February 2019. Retrieved 24 July 2021.
- ↑ "New Drug Therapy Approvals 2019". U.S. Food and Drug Administration. 31 December 2019. Archived from the original on 16 September 2020. Retrieved 15 September 2020.
External links
| External sites: | |
|---|---|
| Identifiers: |
|
- "Drug Trials Snapshot: Evenity". U.S. Food and Drug Administration (FDA). Archived from the original on 3 August 2021. Retrieved 24 July 2021.
- Pages using duplicate arguments in template calls
- CS1 maint: multiple names: authors list
- Use dmy dates from November 2019
- Articles with invalid date parameter in template
- Drugs with non-standard legal status
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Drugs missing an ATC code
- Drug has EMA link
- Drugboxes which contain changes to verified fields
- Drugboxes which contain changes to watched fields
- Drugs that are a monoclonal antibody
- All articles with vague or ambiguous time
- Vague or ambiguous time from November 2019
- Articles with changed CASNo identifier
- Chemicals that do not have a ChemSpider ID assigned
- Articles with changed KEGG identifier
- Amgen
- Monoclonal antibodies
- RTT