Ethinylestradiol/drospirenone
 Ethinylestradiol | |
 Drospirenone | |
| Combination of | |
|---|---|
| Ethinylestradiol | Estrogen |
| Drospirenone | Progestogen; Progestin; Antimineralocorticoid; Antiandrogen |
| Names | |
| Trade names | With 30 μg ethinylestradiol: Yasmin, others With 20 μg ethinylestradiol: Yaz, Yasminelle, others |
| Other names | EE/DRSP |
| Clinical data | |
| Drug class | Estrogen; Progestin; Progestogen; Antimineralocorticoid; Antiandrogen |
| Pregnancy category | |
| Routes of use | By mouth |
| External links | |
| AHFS/Drugs.com | Professional Drug Facts |
| MedlinePlus | a601050 |
| Legal | |
| License data | |
| Legal status |
|
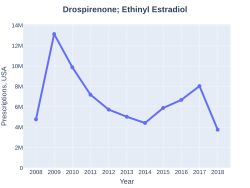
Ethinylestradiol/drospirenone (EE/DRSP), sold under the brand name Yasmin among others, is a combination of ethinylestradiol (EE), an estrogen, and drospirenone (DRSP), a progestin, antimineralocorticoid, and antiandrogen, which is used as a birth control pill to prevent pregnancy in women.[2][3][4][5] It is also indicated for the treatment of moderate acne, premenstrual syndrome (PMS), premenstrual dysphoric disorder (PMDD), and dysmenorrhea (painful menstruation) in women.[3] The medication is taken by mouth and contains 30 μg EE and 3 mg DRSP per tablet (brand names Yasmin, others) or 20 μg EE and 3 mg DRSP per tablet (brand names Yaz, Yasminelle, Nikki, others).[4][5] A formulation with levomefolic acid (vitamin B9) has also been marketed (brand names Beyaz, Safyral, others), with similar indications.[6][7] EE/DRSP is marketed widely throughout the world.[8]In 2017, it was the 98th most commonly prescribed medication in the United States, with more than eight million prescriptions.[9][10]
Society and culture
Cost
In 2017, it was the 98th most commonly prescribed medication in the United States, with more than eight million prescriptions.[9][10]
-
Ethinylestradiol/drospirenone costs (US)
-
Ethinylestradiol/drospirenone prescriptions (US)
See also
- Ethinylestradiol/drospirenone/levomefolic acid
- Ethinylestradiol/drospirenone/prasterone
- Estradiol/drospirenone
- List of combined sex-hormonal preparations § Estrogens and progestogens
References
- ↑ 1.0 1.1 "Drospirenone / estradiol (Angeliq) Use During Pregnancy". Drugs.com. 6 September 2018. Archived from the original on 18 March 2020. Retrieved 18 March 2020.
- ↑ "Archive copy". Archived from the original on 2021-08-28. Retrieved 2021-03-02.
{{cite web}}: CS1 maint: archived copy as title (link) - ↑ 3.0 3.1 "Archive copy". Archived from the original on 2019-12-23. Retrieved 2021-03-02.
{{cite web}}: CS1 maint: archived copy as title (link) - ↑ 4.0 4.1 "Archive copy" (PDF). Archived (PDF) from the original on 2021-04-02. Retrieved 2021-03-02.
{{cite web}}: CS1 maint: archived copy as title (link) - ↑ 5.0 5.1 "Yaz" (PDF). Archived from the original (PDF) on October 31, 2017. Retrieved October 31, 2017.
- ↑ "Archive copy". Archived from the original on 2019-12-23. Retrieved 2021-03-02.
{{cite web}}: CS1 maint: archived copy as title (link) - ↑ "Archive copy" (PDF). Archived (PDF) from the original on 2021-03-30. Retrieved 2021-03-02.
{{cite web}}: CS1 maint: archived copy as title (link) - ↑ "Archive copy". Archived from the original on 2020-11-29. Retrieved 2021-03-02.
{{cite web}}: CS1 maint: archived copy as title (link) - ↑ 9.0 9.1 "The Top 300 of 2020". ClinCalc. Archived from the original on 12 February 2021. Retrieved 11 April 2020.
- ↑ 10.0 10.1 "Drospirenone; Ethinyl Estradiol - Drug Usage Statistics". ClinCalc. Archived from the original on 8 July 2020. Retrieved 11 April 2020.
External links
| Identifiers: |
|---|
- "Drospirenone mixture with estradiol". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 2020-03-18. Retrieved 2021-03-02.
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
This drug article relating to the genito-urinary system is a stub. You can help MDWiki by expanding it. |
- Pages using duplicate arguments in template calls
- CS1 maint: archived copy as title
- Chemical articles with unknown parameter in Infobox drug
- Infobox drug articles with non-default infobox title
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Drugs missing an ATC code
- Articles containing unverified chemical infoboxes
- Drugs that are a combination of chemicals
- Combined oral contraceptives
- Bayer brands
- All stub articles
- Genito-urinary system drug stubs