Rezvilutamide
Jump to navigation
Jump to search
 | |
| Clinical data | |
|---|---|
| Other names | SHR3680 |
| Drug class | Nonsteroidal antiandrogen |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| UNII | |
| Chemical and physical data | |
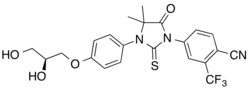
| Formula | C22H20F3N3O4S |
| Molar mass | 479.47 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Rezvilutamide (INN),[1][2] sold under the brand name Ariane, is a nonsteroidal antiandrogen which is approved for the treatment of prostate cancer in China and is or was under development for the treatment of breast cancer.[3][4][5] It is a selective androgen receptor antagonist with reduced brain distribution compared to the structurally related nonsteroidal antiandrogen enzalutamide.[3][5] The drug was developed by Jiangsu Hengrui Medicine.[3] Other structural analogues of rezvilutamide that are also used as antiandrogens besides enzalutamide include apalutamide and proxalutamide.
References
- ^ "Rezvilutamide". chemidplus. U.S. National Library of Medicine.
- ^ "International Nonproprietary Names for Pharmaceutical Substances (INN)" (PDF). WHO.
- ^ a b c "SHR 3680". AdisInsight.
- ^ Keam SJ (January 2023). "Rezvilutamide: First Approval". Drugs. 83 (2): 189–193. doi:10.1007/s40265-022-01831-y. PMID 36630077. S2CID 255593586.
- ^ a b Qin X, Han W, Luo H, Du C, Zou Q, Sun Z, et al. (2020). "SHR3680, a novel antiandrogen, for the treatment of metastatic castration-resistant prostate cancer (mCRPC): A phase I/II study". Journal of Clinical Oncology. 38 (6_suppl): 90. doi:10.1200/JCO.2020.38.6_suppl.90. S2CID 214027454.
Categories:
- Articles with short description
- Short description matches Wikidata
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Drugs missing an ATC code
- Drugs with no legal status
- Articles containing unverified chemical infoboxes
- Benzonitriles
- Diols
- Hormonal antineoplastic drugs
- Ketones
- Imidazolidines
- Nonsteroidal antiandrogens
- Sulfur compounds
- Trifluoromethyl compounds
- Drugs developed by Jiangsu Hengrui
- All stub articles
- Genito-urinary system drug stubs