Cefiderocol
 | |
| Names | |
|---|---|
| Trade names | Fetroja, Fetcroja |
| Other names | Cephiderocol, RSC-649266 |
| |
| Clinical data | |
| Drug class | Siderophore cephalosporin[1] |
| Main uses | Urinary tract infections, hospital acquired pneumonia[2] |
| Side effects | Diarrhea, injection site pain, constipation, rash, candidiasis, liver problems, low potassium[2] |
| WHO AWaRe | |
| Routes of use | Intravenous infusion |
| Typical dose | 2 g TID[3] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a620008 |
| Legal | |
| License data | |
| Legal status |
|
| Pharmacokinetics | |
| Protein binding | 56–58%[4] |
| Elimination half-life | 2.8 hours |
| Excretion | Mainly kidney (60–70% unchanged) |
| Chemical and physical data | |
| Formula | C30H34ClN7O10S2 |
| Molar mass | 752.21 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Cefiderocol, sold under the brand name Fetroja among others, is an antibiotic used to treat complicated urinary tract infections and hospital acquired pneumonia.[2] It is used when other treatments might not work, including for Pseudomonas aeruginosa.[3][2] It is given by injection into a vein.[2]
Common side effects include diarrhea, pain at the site of injection, constipation, rash, candidiasis, liver problems, and low potassium.[2] Other side effects may include anaphylaxis, Clostridioides difficile infection, and seizures.[2] Safety in pregnancy is unclear.[2] It is in the cephalosporin family of medications.[2]
Cefiderocol was approved for medical use in the United States in 2019 and Europe in 2020.[2][3] It is on the World Health Organization's List of Essential Medicines.[5] In the United Kingdom 5 doses costs about £1,300.[6] This amount in the United States is about 2,000 USD.[7]
Medical uses
Cefiderocol is used to treat adults with complicated urinary tract infections, including kidney infections caused by susceptible Gram-negative microorganisms, who have limited or no alternative treatment options.[8][9] It is used to treat multi-drug-resistant Gram-negative bacteria including Pseudomonas aeruginosa.[10]
In the United States, cefiderocol is indicated in adults 18 years of age or older who have limited or no alternative treatment options for the treatment of complicated urinary tract infections (cUTIs), including pyelonephritis caused by the following susceptible Gram-negative microorganisms: Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, Pseudomonas aeruginosa, and Enterobacter cloacae complex.[2]
For the treatment of severe pneumonia (HABP and VABP), it is indicated in patients 18 years of age and older whose pneumonia is not responding to other, more commonly used antibiotics and is confirmed to be caused by one of the following Gram-negative organisms:
- Acinetobacter baumannii complex
- Escherichia coli
- Enterobacter cloacae complex
- Klebsiella pneumoniae
- Pseudomonas aeruginosa
- Serratia marcescens
This indication is supported by the APEKS-NP study,[11] where cefiderocol was compared to meropenem (a widely used antibiotic for multidrug-resistant bacteria causing pneumonia, among other diseases), where it was shown not to be inferior to meropenem. The primary endpoint of the study was all-cause mortality at day 14, where both antibiotics were shown to be almost equally effective.
In the European Union, cefiderocol is indicated for the treatment of infections due to aerobic Gram-negative organisms in adults with limited treatment options.[3]
Dosage
It is generally used at a dose of 2 g given every 8 hours.[3]
Side effects
An increased risk of dying was observed in people treated with cefiderocol as compared to other antibiotics in a separate clinical trial.[12] Most of the deaths occurred in people hospitalized with other kinds of severe bacterial infections (pneumonia, sepsis, or infection in the blood).[12] The cause of death was not clear.[12]
Cefiderocol may cause serious and life-threatening allergic reactions, severe diarrhea caused by C. difficile and seizures.[12]
Labeling for cefiderocol includes a warning regarding the higher all-cause mortality rate observed in cefiderocol-treated people compared to those treated with other antibiotics in a trial in critically ill people with multidrug-resistant Gram-negative bacterial infections.[8] The cause of the increase in mortality has not been established.[8] Some of the deaths were a result of worsening or complications of infection, or underlying co-morbidities.[8] The higher all-cause mortality rate was observed in people treated for hospital-acquired/ventilator-associated pneumonia (i.e.nosocomial pneumonia), bloodstream infections, or sepsis.[8] The safety and efficacy of cefiderocol has not been established for the treatment of these types of infections.[8]
Pharmacology
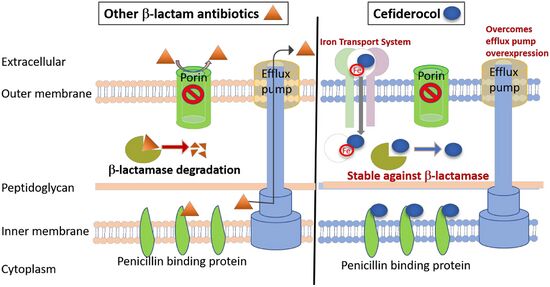
Mechanism of action
Its mechanism of entry into bacterial cells is by binding to iron, which is actively transported into the bacterial cells along with the cefiderocol.[2][14][15][16][17]
It is in a medication class known as siderophores,[2][9] and was the first siderophore antibiotic to be approved by the U.S. Food and Drug Administration (FDA).[18]
It bypasses the bacterial porin channels by using the bacteria's own iron-transport system for being transported in.[19]
History
In 2019, cefiderocol was approved in the United States as an antibacterial drug for treatment of adults 18 years of age or older with complicated urinary tract infections (cUTI), including kidney infections caused by susceptible Gram-negative microorganisms, who have limited or no alternative treatment options.[8][20][2]
The safety and effectiveness of cefiderocol was demonstrated in a study (NCT02321800) of 448 participants with cUTIs.[8][12] Of the participants who were administered cefiderocol, 72.6% had resolution of symptoms and eradication of the bacteria approximately seven days after completing treatment, compared with 54.6% in participants who received an alternative antibiotic.[8] The clinical response rates were similar between the two treatment groups.[8] The trial included participants from Europe, United States and Mexico.[12]
In the clinical trial, participants with cUTI were chosen at random to receive cefiderocol, or another antibacterial drug called imipenem/cilastatin.[12] Both treatments were given intravenously for 7–14 days and neither the participants nor the health care professionals knew which drugs were given until after the trial was complete.[12] Participants could not be switched to an oral antibacterial drug to complete the treatment for cUTI.[12]
The benefit of cefiderocol was measured by the proportion of participants who achieved cure or improvement in their symptoms related to cUTI and a negative urine culture test in comparison to imipenem/cilastatin.[12]
Cefiderocol received a Qualified Infectious Disease Product designation from the U.S. Food and Drug Administration (FDA) and was granted priority review.[8] The FDA granted approval of Fetroja, on 14 November 2019, to Shionogi & Co., Ltd.[8]
Cefiderocol was approved for medical use in the European Union in April 2020.[3]
References
- ↑ "Cefiderocol Monograph for Professionals". Drugs.com. Archived from the original on 21 January 2021. Retrieved 30 December 2021.
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 2.13 "Fetroja- cefiderocol sulfate tosylate injection, powder, for solution". DailyMed. 19 November 2019. Archived from the original on 1 February 2021. Retrieved 29 April 2020.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 "Fetcroja EPAR". European Medicines Agency (EMA). 24 February 2020. Archived from the original on 17 September 2020. Retrieved 29 April 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ Katsube, T.; Echols, R.; Arjona Ferreira, J. C.; et al. (2017). "Cefiderocol, a Siderophore Cephalosporin for Gram‐Negative Bacterial Infections: Pharmacokinetics and Safety in Subjects With Renal Impairment". Journal of Clinical Pharmacology. 57 (5): 584–591. doi:10.1002/jcph.841. PMC 5412848. PMID 27874971.
- ↑ World Health Organization (2021). World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. hdl:10665/345533. WHO/MHP/HPS/EML/2021.02.
- ↑ BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 562. ISBN 978-0857114105.
- ↑ "Fetroja Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 22 May 2023. Retrieved 30 December 2021.
- ↑ 8.00 8.01 8.02 8.03 8.04 8.05 8.06 8.07 8.08 8.09 8.10 8.11 "FDA approves new antibacterial drug to treat complicated urinary tract infections as part of ongoing efforts to address antimicrobial resistance". U.S. Food and Drug Administration (FDA) (Press release). 14 November 2019. Archived from the original on 16 November 2019. Retrieved 15 November 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ 9.0 9.1 Zhanel GG, Golden AR, Zelenitsky S, et al. (February 2019). "Cefiderocol: A Siderophore Cephalosporin with Activity Against Carbapenem-Resistant and Multidrug-Resistant Gram-Negative Bacilli". Drugs. 79 (3): 271–289. doi:10.1007/s40265-019-1055-2. PMID 30712199. S2CID 59541210.
- ↑ Choi, Justin J; McCarthy, Matthew W. (24 January 2018). "Cefiderocol: a novel siderophore cephalosporin". Expert Opinion on Investigational Drugs. 27 (2): 193–197. doi:10.1080/13543784.2018.1426745. PMID 29318906. S2CID 205768562.
- ↑ "Cefiderocol (S-649266) for Nosocomial Pneumonia Caused by Gram-Negative Pathogens: Study Design of APEKS-NP, a Phase 3 Double-Blind Parallel-Group Randomized Clinical Trial". American Thoracic Society International Conference Abstracts. American Thoracic Society. May 2018: A3290. doi:10.1164/ajrccm-conference.2018.197.1_MeetingAbstracts.A3290 (inactive 31 May 2021). Archived from the original on 18 February 2022. Retrieved 20 September 2020.
{{cite journal}}: Cite journal requires|journal=(help)CS1 maint: DOI inactive as of May 2021 (link) - ↑ 12.0 12.1 12.2 12.3 12.4 12.5 12.6 12.7 12.8 12.9 "Drug Trials Snapshot: Fetroja". U.S. Food and Drug Administration (FDA). 14 November 2019. Archived from the original on 6 August 2020. Retrieved 29 April 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ Wang, Hongmei; Palasik, Brittany N. (January 2022). "Combating antimicrobial resistance with cefiderocol for complicated infections involving the urinary tract". Therapeutic Advances in Urology. 14: 175628722110655. doi:10.1177/17562872211065570. ISSN 1756-2872.
- ↑ Sato T, Yamawaki K (November 2019). "Cefiderocol: Discovery, Chemistry, and In Vivo Profiles of a Novel Siderophore Cephalosporin". Clin. Infect. Dis. 69 (Supplement_7): S538–S543. doi:10.1093/cid/ciz826. PMC 6853759. PMID 31724047.
- ↑ Matthews-King A (2018-10-26). "Antibiotic 'Trojan horse' could defeat superbugs causing global medical crisis, trial finds". The Independent. Archived from the original on 2021-10-09. Retrieved 2018-10-26.
- ↑ Newey S (2018-10-26). "New 'Trojan horse' drug proves effective against antibiotic resistant bacteria". The Telegraph. ISSN 0307-1235. Archived from the original on 2019-04-12. Retrieved 2018-10-26.
- ↑ Simpson DH, Scott P (2017). "Antimicrobial Metallodrugs". In Lo K (ed.). Inorganic and Organometallic Transition Metal Complexes with Biological Molecules and Living Cells. Elsevier. ISBN 9780128038871. Archived from the original on 2021-02-25. Retrieved 2021-10-12.
- ↑ Saisho, Yutaka; Katsube, Takayuki; White, Scott; et al. (March 2018). "Pharmacokinetics, Safety, and Tolerability of Cefiderocol, a Novel Siderophore Cephalosporin for Gram-Negative Bacteria, in Healthy Subjects". Antimicrobial Agents and Chemotherapy. 62 (3): 1–12. doi:10.1128/AAC.02163-17. PMC 5826143. PMID 29311072.
- ↑ Ito A, Nishikawa T, Matsumoto S, et al. (December 2016). "Siderophore Cephalosporin Cefiderocol Utilizes Ferric Iron Transporter Systems for Antibacterial Activity against Pseudomonas aeruginosa". Antimicrobial Agents and Chemotherapy. 60 (12): 7396–7401. doi:10.1128/AAC.01405-16. PMC 5119021. PMID 27736756.
- ↑ "Drug Approval Package: Fetroja (cefiderocol)". U.S. Food and Drug Administration (FDA). 19 December 2019. Archived from the original on 5 April 2021. Retrieved 29 April 2020.
External links
| External sites: | |
|---|---|
| Identifiers: |
|
- "Cefiderocol sulfate tosylate". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 2021-11-01. Retrieved 2021-10-12.
- Committee for Medicinal Products for Human Use (27 February 2020). "Fetcroja : EPAR - Public assessment report" (PDF). European Medicines Agency (EMA). EMA/136096/2020. Archived (PDF) from the original on 1 November 2021. Retrieved 12 October 2021.
- Pages using duplicate arguments in template calls
- Wikipedia articles incorporating the PD-notice template
- CS1 errors: missing periodical
- CS1 maint: DOI inactive as of May 2021
- Drugs with non-standard legal status
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without UNII source
- Drugs missing an ATC code
- Drug has EMA link
- Articles containing unverified chemical infoboxes
- Cephalosporin antibiotics
- Siderophores
- World Health Organization essential medicines
- RTT