Cisplatin
| |||
| Names | |||
|---|---|---|---|
| Trade names | Platinol, others | ||
| Other names | Cisplatinum, platamin, neoplatin, cismaplat, cis-diamminedichloridoplatinum(II) (CDDP) | ||
| |||
| Clinical data | |||
| Drug class | Chemotherapy | ||
| Main uses | Cancers[1] | ||
| Side effects | Bone marrow suppression, hearing problems, kidney problems, vomiting[1][2] | ||
| Pregnancy category | |||
| Routes of use | Intravenous | ||
| Defined daily dose | not established[4] | ||
| External links | |||
| AHFS/Drugs.com | Monograph | ||
| MedlinePlus | a684036 | ||
| Legal | |||
| License data | |||
| Legal status | |||
| Pharmacokinetics | |||
| Bioavailability | 100% (IV) | ||
| Protein binding | > 95% | ||
| Elimination half-life | 30–100 hours | ||
| Excretion | Renal | ||
| Chemical and physical data | |||
| Formula | [Pt(NH3)2Cl2] | ||
| Molar mass | 300.05 g·mol−1 | ||
| 3D model (JSmol) | |||
| |||
| |||
Cisplatin is a chemotherapy medication used to treat a number of cancers.[1] These include testicular cancer, ovarian cancer, cervical cancer, breast cancer, bladder cancer, head and neck cancer, esophageal cancer, lung cancer, mesothelioma, brain tumors and neuroblastoma.[1] It is given by injection into a vein.[1]
Common side effects include bone marrow suppression, hearing problems, kidney problems, and vomiting.[1][2] Other serious side effects include numbness, trouble walking, allergic reactions, electrolyte problems, and heart disease.[1] Use during pregnancy can cause harm to the baby.[3][1] Cisplatin is in the platinum-based antineoplastic family of medications.[1] It works in part by binding to DNA and inhibiting its replication.[1]
Cisplatin was discovered in 1845 and licensed for medical use in 1978 and 1979.[5][1] It is on the World Health Organization's List of Essential Medicines.[6] The wholesale cost in the developing world is about US$5.56 to US$7.98 per 50 mg vial.[7] In the United Kingdom this costs the NHS about £17.[8]
Medical use
Cisplatin is administered intravenously as short-term infusion in normal saline for treatment of solid and haematological malignancies. It is used to treat various types of cancers, including sarcomas, some carcinomas (e.g., small cell lung cancer, squamous cell carcinoma of the head and neck and ovarian cancer), lymphomas, bladder cancer, cervical cancer,[9] and germ cell tumors.
Cisplatin is particularly effective against testicular cancer; its adoption has increased the cure rate from 10% to 85%.[10]
In addition, cisplatin is used in Auger therapy.[medical citation needed]
Dosage
The defined daily dose is not established[4]
Side effects
Cisplatin has a number of side effects that can limit its use:
- Nephrotoxicity (kidney damage) is a major concern.[1] The dose should be reduced when the person's kidney function is impaired. Adequate hydration is used in an effort to prevent damage.[1] Amifostine has been studied in an effort to prevent problems.[11] Nephrotoxicity is a dose-limiting side effect.[1]
- Neurotoxicity (nerve damage) can be anticipated by performing nerve conduction studies before and after treatment. Common neurological side effects of cisplatin include visual perception and hearing disorder, which can occur soon after treatment begins.[12] While triggering apoptosis through interfering with DNA replication remains the primary mechanism of cisplatin, this has not been found to contribute to neurological side effects. Recent studies have shown that cisplatin noncompetitively inhibits an archetypal, membrane-bound mechanosensitive sodium-hydrogen ion transporter known as NHE-1.[12] It is primarily found on cells of the peripheral nervous system, which are aggregated in large numbers near the ocular and aural stimuli-receiving centers. This noncompetitive interaction has been linked to hydroelectrolytic imbalances and cytoskeleton alterations, both of which have been confirmed in vitro and in vivo. However, NHE-1 inhibition has been found to be both dose-dependent (half-inhibition = 30 μg/mL) and reversible.[12]
- Nausea and vomiting: cisplatin is one of the most emetogenic chemotherapy agents, but this symptom is managed with prophylactic antiemetics (ondansetron, granisetron, etc.) in combination with corticosteroids. Aprepitant combined with ondansetron and dexamethasone has been shown to be better for highly emetogenic chemotherapy than just ondansetron and dexamethasone.
- Ototoxicity (hearing loss): there is at present no effective treatment to prevent this side effect, which may be severe. Audiometric analysis may be necessary to assess the severity of ototoxicity. Other drugs (such as the aminoglycoside antibiotic class) may also cause ototoxicity, and the administration of this class of antibiotics in patients receiving cisplatin is generally avoided. The ototoxicity of both the aminoglycosides and cisplatin may be related to their ability to bind to melanin in the stria vascularis of the inner ear or the generation of reactive oxygen species.
- Electrolyte disturbance: Cisplatin can cause hypomagnesaemia, hypokalaemia and hypocalcaemia. The hypocalcaemia seems to occur in those with low serum magnesium secondary to cisplatin, so it is not primarily due to the cisplatin.
- Hemolytic anemia can be developed after several courses of cisplatin. It is suggested that an antibody reacting with a cisplatin-red-cell membrane is responsible for hemolysis.[13]
Pharmacology
Cisplatin interferes with DNA replication, which kills the fastest proliferating cells, which in theory are cancerous. Following administration, one chloride ion is slowly displaced by water to give the aquo complex cis-[PtCl(NH3)2(H2O)]+, in a process termed aquation. Dissociation of the chloride is favored inside the cell because the intracellular chloride concentration is only 3–20% of the approximately 100 mM chloride concentration in the extracellular fluid.[14][15]
The water molecule in cis-[PtCl(NH3)2(H2O)]+ is itself easily displaced by the N-heterocyclic bases on DNA. Guanine preferentially binds. Subsequent to formation of [PtCl(guanine-DNA)(NH3)2]+, crosslinking can occur via displacement of the other chloride, typically by another guanine.[16] Cisplatin crosslinks DNA in several different ways, interfering with cell division by mitosis. The damaged DNA elicits DNA repair mechanisms, which in turn activate apoptosis when repair proves impossible. In 2008, researchers were able to show that the apoptosis induced by cisplatin on human colon cancer cells depends on the mitochondrial serine-protease Omi/Htra2.[17] Since this was only demonstrated for colon carcinoma cells, it remains an open question if the Omi/Htra2 protein participates in the cisplatin-induced apoptosis in carcinomas from other tissues.[medical citation needed]
Most notable among the changes in DNA are the 1,2-intrastrand cross-links with purine bases. These include 1,2-intrastrand d(GpG) adducts which form nearly 90% of the adducts and the less common 1,2-intrastrand d(ApG) adducts. 1,3-intrastrand d(GpXpG) adducts occur but are readily excised by the nucleotide excision repair (NER). Other adducts include inter-strand crosslinks and nonfunctional adducts that have been postulated to contribute to cisplatin's activity. Interaction with cellular proteins, particularly HMG domain proteins, has also been advanced as a mechanism of interfering with mitosis, although this is probably not its primary method of action.[medical citation needed]
Cisplatin resistance

Cisplatin combination chemotherapy is the cornerstone of treatment of many cancers. Initial platinum responsiveness is high but the majority of cancer patients will eventually relapse with cisplatin-resistant disease. Many mechanisms of cisplatin resistance have been proposed including changes in cellular uptake and efflux of the drug, increased detoxification of the drug, inhibition of apoptosis and increased DNA repair.[19]
Oxaliplatin is active in highly cisplatin-resistant cancer cells in the laboratory; however, there is little evidence for its activity in the clinical treatment of patients with cisplatin-resistant cancer.[19] The drug paclitaxel may be useful in the treatment of cisplatin-resistant cancer; the mechanism for this activity is unknown.[20]
Transplatin
Transplatin, the trans stereoisomer of cisplatin, has formula trans-[PtCl2(NH3)2] and does not exhibit a comparably useful pharmacological effect. Two mechanisms have been suggested to explain the reduced anticancer effect of transplatin. Firstly, the trans arrangement of the chloro ligands is thought to confer transplatin with greater chemical reactivity, causing transplatin to become deactivated before it reaches the DNA where cisplatin exerts its pharmacological action. Secondly, the stereo-conformation of transplatin is such that it is unable to form the characteristic 1,2-intrastrand d(GpG) adducts formed by cisplatin in abundance.[21]
Molecular structure
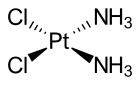
Cisplatin is the square planar coordination complex cis-[Pt(NH3)2Cl2].[22]: 286–8 [23]: 689 The prefix cis indicates the cis isomer in which two similar ligands are in adjacent positions.[22][23]: 550 The systematic chemical name of this molecule is cis–diamminedichloroplatinum, where ammine with two m's indicates an ammonia (NH3) ligand, as opposed to an organic amine with one m.[22]: 284
History
The compound cis-[Pt(NH3)2Cl2] was first described by Michele Peyrone in 1845, and known for a long time as Peyrone's salt.[24] The structure was deduced by Alfred Werner in 1893.[16] In 1965, Barnett Rosenberg, Van Camp et al. of Michigan State University discovered that electrolysis of platinum electrodes generated a soluble platinum complex which inhibited binary fission in Escherichia coli (E. coli) bacteria. Although bacterial cell growth continued, cell division was arrested, the bacteria growing as filaments up to 300 times their normal length.[25] The octahedral Pt(IV) complex cis-[PtCl4(NH3)2], but not the trans isomer, was found to be effective at forcing filamentous growth of E. coli cells. The square planar Pt(II) complex, cis-[PtCl2(NH3)2] turned out to be even more effective at forcing filamentous growth.[26][27] This finding led to the observation that cis-[PtCl2(NH3)2] was indeed highly effective at regressing the mass of sarcomas in rats.[28] Confirmation of this discovery, and extension of testing to other tumour cell lines launched the medicinal applications of cisplatin. Cisplatin was approved for use in testicular and ovarian cancers by the U.S. Food and Drug Administration on 19 December 1978.,[16][29][30] and in the UK (and in several other European countries) in 1979.[31]
Synthesis
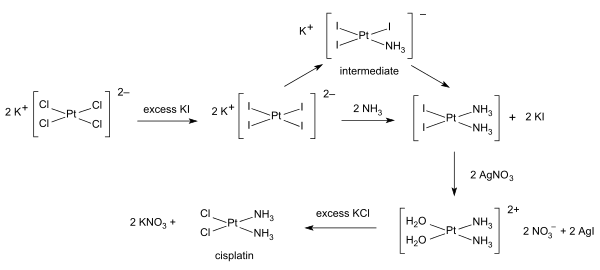
Syntheses of cisplatin start from potassium tetrachloroplatinate. Several procedures are available. One obstacle is the facile formation of Magnus's green salt (MGS), which has the same empirical formula as cisplatin. The traditional way to avoid MGS involves the conversion of K2PtCl4 to K2PtI4, as originally described by Dhara.[32][33] Reaction with ammonia forms K2[PtI2(NH3)2] which is isolated as a yellow compound. When silver nitrate in water is added insoluble silver iodide precipitates and K2[Pt(OH2)2(NH3)2] remains in solution. Addition of potassium chloride will form the final product which precipitates [33] In the triiodo intermediate the addition of the second ammonia ligand is governed by the trans effect.[33]
A one-pot synthesis of cisplatin from K2PtCl4 has been developed. It relies on the slow release of ammonia from ammonium acetate.[34]
See also
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 "Cisplatin". The American Society of Health-System Pharmacists. Archived from the original on 21 December 2016. Retrieved 8 December 2016.
- ↑ 2.0 2.1 Oun R, Moussa YE, Wheate NJ (May 2018). "The side effects of platinum-based chemotherapy drugs: a review for chemists". Dalton Transactions. 47 (19): 6645–6653. doi:10.1039/c8dt00838h. PMID 29632935.
- ↑ 3.0 3.1 3.2 3.3 "Cisplatin Use During Pregnancy". Drugs.com. 12 September 2019. Archived from the original on 3 December 2020. Retrieved 25 February 2020.
- ↑ 4.0 4.1 "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 21 October 2020. Retrieved 17 September 2020.
- ↑ Fischer, Janos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 513. ISBN 9783527607495. Archived from the original on 20 December 2016.
- ↑ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ↑ "Cisplatin". International Drug Price Indicator Guide. Archived from the original on 22 January 2018. Retrieved 8 December 2016.
- ↑ British national formulary: BNF 69 (69 ed.). British Medical Association. 2015. p. 605. ISBN 9780857111562.
- ↑ "Cisplatin". National Cancer Institute. Archived from the original on 8 October 2014. Retrieved 13 November 2014.
- ↑ Einhorn LH (November 1990). "Treatment of testicular cancer: a new and improved model". Journal of Clinical Oncology. 8 (11): 1777–81. doi:10.1200/JCO.1990.8.11.1777. PMID 1700077.
- ↑ Avan A, Postma TJ, Ceresa C, Avan A, Cavaletti G, Giovannetti E, Peters GJ (April 2015). "Platinum-induced neurotoxicity and preventive strategies: past, present, and future". The Oncologist. 20 (4): 411–32. doi:10.1634/theoncologist.2014-0044. PMC 4391771. PMID 25765877.
- ↑ 12.0 12.1 12.2 Milosavljevic N, Duranton C, Djerbi N, Puech PH, Gounon P, Lagadic-Gossmann D, et al. (October 2010). "Nongenomic effects of cisplatin: acute inhibition of mechanosensitive transporters and channels without actin remodeling". Cancer Research. 70 (19): 7514–22. doi:10.1158/0008-5472.CAN-10-1253. PMID 20841472.
- Lay summary in: "Elucidating side effects of antineoplastic agent". ScienceDaily.
- ↑ Levi JA, Aroney RS, Dalley DN (June 1981). "Haemolytic anaemia after cisplatin treatment". British Medical Journal. 282 (6281): 2003–4. doi:10.1136/bmj.282.6281.2003. PMC 1505958. PMID 6788166.
- ↑ Wang D, Lippard SJ (April 2005). "Cellular processing of platinum anticancer drugs". Nature Reviews. Drug Discovery. 4 (4): 307–20. doi:10.1038/nrd1691. PMID 15789122. S2CID 31357727.
- ↑ Johnstone TC, Suntharalingam K, Lippard SJ (March 2016). "The Next Generation of Platinum Drugs: Targeted Pt(II) Agents, Nanoparticle Delivery, and Pt(IV) Prodrugs". Chemical Reviews. 116 (5): 3436–86. doi:10.1021/acs.chemrev.5b00597. PMC 4792284. PMID 26865551.
- ↑ 16.0 16.1 16.2 Trzaska, Stephen (20 June 2005). "Cisplatin". Chemical & Engineering News. 83 (25): 52. doi:10.1021/cen-v083n025.p052. Archived from the original on 20 April 2017. Retrieved 15 July 2005.
- ↑ Pruefer FG, Lizarraga F, Maldonado V, Melendez-Zajgla J (June 2008). "Participation of Omi Htra2 serine-protease activity in the apoptosis induced by cisplatin on SW480 colon cancer cells". Journal of Chemotherapy. 20 (3): 348–54. doi:10.1179/joc.2008.20.3.348. PMID 18606591. S2CID 11052459.
- ↑ Lugones, Yuliannis; Loren, Pía; Salazar, Luis A. (October 2022). "Cisplatin Resistance: Genetic and Epigenetic Factors Involved". Biomolecules. 12 (10): 1365. doi:10.3390/biom12101365. ISSN 2218-273X.
- ↑ 19.0 19.1 Stordal B, Davey M (November 2007). "Understanding cisplatin resistance using cellular models" (PDF). IUBMB Life. 59 (11): 696–9. doi:10.1080/15216540701636287. PMID 17885832. Archived (PDF) from the original on 28 February 2021. Retrieved 30 November 2019.
- ↑ Stordal B, Pavlakis N, Davey R (December 2007). "A systematic review of platinum and taxane resistance from bench to clinic: an inverse relationship" (PDF). Cancer Treatment Reviews. 33 (8): 688–703. doi:10.1016/j.ctrv.2007.07.013. hdl:2123/4068. PMID 17881133. Archived (PDF) from the original on 28 February 2021. Retrieved 30 November 2019.
- ↑ Coluccia M, Natile G (January 2007). "Trans-platinum complexes in cancer therapy". Anti-Cancer Agents in Medicinal Chemistry. 7 (1): 111–23. doi:10.2174/187152007779314080. PMID 17266508.
- ↑ 22.0 22.1 22.2 Miessler, Gary L.; Tarr, Donald A. (1999). Inorganic Chemistry (2nd ed.). Prentice Hall. ISBN 978-0-13-841891-5.
- ↑ 23.0 23.1 Housecroft, Catherine E.; Sharpe, Alan G. (2005). Inorganic Chemistry (2nd ed.). Pearson Prentice Hall. ISBN 978-0-130-39913-7.
- ↑ Peyrone M (1844). "Ueber die Einwirkung des Ammoniaks auf Platinchlorür" [On the action of ammonia on platinum chloride]. Ann. Chem. Pharm. 51 (1): 1–29. doi:10.1002/jlac.18440510102. Archived from the original on 5 March 2021. Retrieved 29 June 2019.
- ↑ Rosenberg B, Vancamp L, Krigas T (February 1965). "Inhibition of cell division in Escherichia coli by electrolysis products from a platinum electrode". Nature. 205 (4972): 698–9. Bibcode:1965Natur.205..698R. doi:10.1038/205698a0. PMID 14287410.
- ↑ Rosenberg B, Van Camp L, Grimley EB, Thomson AJ (March 1967). "The inhibition of growth or cell division in Escherichia coli by different ionic species of platinum(IV) complexes". The Journal of Biological Chemistry. 242 (6): 1347–52. PMID 5337590.
- ↑ Christie DA, Tansey EM, Thomson AJ, eds. (2007). The Discovery, Use and Impact of Platinum Salts as Chemotherapy Agent for Cancer. Wellcome Trust Witnesses to Twentieth Century Medicine. Vol. 30. pp. 6–15. ISBN 978-0-85484-112-7.
- ↑ Rosenberg B, VanCamp L, Trosko JE, Mansour VH (April 1969). "Platinum compounds: a new class of potent antitumour agents". Nature. 222 (5191): 385–6. Bibcode:1969Natur.222..385R. doi:10.1038/222385a0. PMID 5782119. S2CID 32398470.
- ↑ Carpenter DP (2010). Reputation and power: organizational image and pharmaceutical regulation at the FDA. Princeton, NJ: Princeton University Press. ISBN 978-0-691-14180-0.
- ↑ "Approval Summary for cisplatin for Metastatic ovarian tumors". FDA Oncology Tools. Food and Drug Administration, Center for Drug Evaluation and Research. 19 December 1978. Archived from the original on 8 February 2008. Retrieved 15 July 2009.
- ↑ Wiltshaw E (1979). "Cisplatin in the treatment of cancer". Platinum Metals Review. 23 (3): 90–8.
- ↑ Dhara SC (1970). "Cisplatin". Indian J. Chem. 8: 123–134.
- ↑ 33.0 33.1 33.2 Alderden RA, Hall MD, Hambley TW (2006). "The Discovery and Development of Cisplatin". J. Chem. Educ. 83 (5): 728. Bibcode:2006JChEd..83..728A. doi:10.1021/ed083p728. S2CID 29546931.
- ↑ Kukushikin, Vadim Yu.; Oskarsson, Åke; Elding, Lars I.; Farrell, Nicholas (2007). Facile Synthesis of Isomerically Pure cis -Dichlorodiammineplatinum(II), Cisplatin. Inorganic Syntheses. Vol. 32. pp. 141–144. doi:10.1002/9780470132630.ch23. ISBN 9780470132630.
Further reading
- Riddell, Imogen A.; Lippard, Stephen J. (2018). "Cisplatin and Oxaliplatin: Our Current Understanding of Their Actions". In Sigel, Astrid; Sigel, Helmut; Freisinger, Eva; Sigel, Roland K. O. (eds.). Metallo-Drugs: Development and Action of Anticancer Agents. Metal Ions in Life Sciences. Vol. 18. Berlin: de Gruyter GmbH. pp. 1–42. doi:10.1515/9783110470734-007. ISBN 978-3-11-046984-4. PMID 29394020.
External links
| External sites: | |
|---|---|
| Identifiers: |
|
- IARC Monograph: "Cisplatin" Archived 11 June 2011 at the Wayback Machine
- Pages using duplicate arguments in template calls
- Use dmy dates from July 2013
- Articles with invalid date parameter in template
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without UNII source
- Drugs missing an ATC code
- Drugboxes which contain changes to verified fields
- Drugboxes which contain changes to watched fields
- All articles with unsourced statements
- Articles with unsourced statements from February 2020
- Articles with changed ChemSpider identifier
- Articles with changed EBI identifier
- Webarchive template wayback links
- Platinum compounds
- Ammine complexes
- Bioinorganic chemistry
- Cancer treatments
- Chemotherapy
- Chloro complexes
- Coordination compounds
- IARC Group 2A carcinogens
- Medicinal inorganic chemistry
- Metal-containing drugs
- Nephrotoxins
- Platinum complexes
- Platinum-based antineoplastic agents
- Platinum(II) compounds
- World Health Organization essential medicines
- RTT