Alpelisib
 | |
| Names | |
|---|---|
| Trade names | Piqray |
| Other names | BYL719 |
| |
| Clinical data | |
| Drug class | Chemotherapy (PI3K inhibitor)[1] |
| Main uses | Certain types of breast cancer[1] |
| Side effects | High blood sugar, kidney problems, diarrhea, rash, low blood cells, liver problems, pancreatitis, vomiting, hair loss[2] |
| Pregnancy category | |
| Routes of use | By mouth |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a619036 |
| Legal | |
| License data | |
| Legal status | |
| Chemical and physical data | |
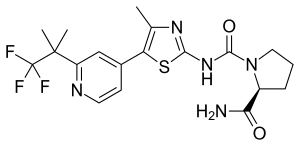
| Formula | C19H22F3N5O2S |
| Molar mass | 441.47 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Alpelisib, sold under the brand name Piqray, is a chemotherapy medication used to treat certain types of breast cancer.[1] It is used together with fulvestrant.[1] It results in about an extra 5 months of life without the disease getting worse.[5] It is taken by mouth.[1]
Common side effects include high blood sugar, kidney problems, diarrhea, rash, low blood cells, liver problems, pancreatitis, vomiting, and hair loss.[2] Other side effects may include anaphylaxis, infertility, and pneumonitis.[1] Use during pregnancy may harm the baby.[1] It is an alpha-specific PI3K inhibitor.[1]
Alpelisib was approved for medical use in the United States in 2019 and Europe in 2020.[1][5] In the United States a month of medication costs about 17,000 USD as of 2021.[7]
Medical uses
Alpelisib is used in combination with fulvestrant for the treatment of postmenopausal women, and men, with hormone receptor (HR)‑positive, human epidermal growth factor receptor 2 (HER2)‑negative, locally advanced or metastatic breast cancer with a PIK3CA mutation after disease progression following endocrine therapy as monotherapy.[5][2]
Dosage
The dose is 300 mg twice per day, until no longer effective or side effects become to great.[1]
History
In May 2019, alpelisib was approved in the United States for use in combination with the endocrine therapy fulvestrant, to treat postmenopausal women, and men, with hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative, PIK3CA-mutated, advanced or metastatic breast cancer following progression on or after an endocrine-based regimen.[2][6][8]
The U.S. Food and Drug Administration (FDA) also approved the companion diagnostic test, therascreen PIK3CA RGQ PCR Kit, to detect the PIK3CA mutation in a tissue and/or a liquid biopsy.[2]
The efficacy of alpelisib was studied in the SOLAR-1 trial (NCT02437318), a randomized trial of 572 postmenopausal women and men with HR-positive, HER2-negative, advanced or metastatic breast cancer whose cancer had progressed while on or after receiving an aromatase inhibitor.[2][9]
The FDA granted the application for alpelisib priority review designation and granted approval of Piqray to Novartis.[2] The FDA granted approval of the therascreen PIK3CA RGQ PCR Kit to Qiagen Manchester, Ltd.[2]
On 28 May 2020, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) adopted a positive opinion, recommending the granting of a marketing authorization for the medicinal product alpelisib (Piqray), intended for the treatment of locally advanced or metastatic breast cancer with a PIK3CA mutation.[5] The applicant for this medicinal product is Novartis Europharm Limited.[5] Alpelisib was approved for medical use in the European Union in July 2020.[5]
Society and culture
Legal status
Alpelisib was approved for medical use in the United States in May 2019,[2][8] in Australia in March 2020,[10] and in the European Union in July 2020.[5]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 1.9 "Alpelisib Monograph for Professionals". Drugs.com. Archived from the original on 4 November 2019. Retrieved 19 July 2021.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 2.8 "FDA approves first PI3K inhibitor for breast cancer". U.S. Food and Drug Administration (FDA) (Press release). 24 May 2019. Archived from the original on 25 November 2019. Retrieved 29 May 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ 3.0 3.1 "Piqray Australian prescription medicine decision summary". Therapeutic Goods Administration (TGA). 27 March 2020. Archived from the original on 27 August 2021. Retrieved 16 August 2020.
- ↑ "Alpelisib (Piqray) Use During Pregnancy". Drugs.com. 29 July 2019. Archived from the original on 3 December 2020. Retrieved 16 August 2020.
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 5.6 5.7 "Piqray EPAR". European Medicines Agency (EMA). 26 May 2020. Archived from the original on 14 August 2020. Retrieved 16 August 2020. Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ↑ 6.0 6.1 "Piqray- alpelisib tablet Piqray- alpelisib kit". DailyMed. 12 June 2020. Archived from the original on 28 October 2020. Retrieved 16 August 2020.
- ↑ "Piqray Prices, Coupons & Savings Tips - GoodRx". GoodRx. Archived from the original on 6 October 2021. Retrieved 19 July 2021.
- ↑ 8.0 8.1 "Drug Approval Package: Piqray". U.S. Food and Drug Administration (FDA). 18 June 2019. Archived from the original on 25 November 2019. Retrieved 25 November 2019.
- ↑ "Drug Trials Snapshots: Piqray". U.S. Food and Drug Administration (FDA). 14 June 2019. Archived from the original on 25 November 2019. Retrieved 24 November 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "AusPAR: Alpelisib". Therapeutic Goods Administration (TGA). 3 September 2020. Archived from the original on 27 October 2020. Retrieved 23 September 2020.
External links
| External sites: | |
|---|---|
| Identifiers: |
|
- Pages using duplicate arguments in template calls
- Wikipedia articles incorporating the PD-notice template
- Use dmy dates from November 2019
- Articles with invalid date parameter in template
- Drugs with non-standard legal status
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without UNII source
- Drugs missing an ATC code
- Drug has EMA link
- Articles containing unverified chemical infoboxes
- Amides
- Novartis brands
- Phosphoinositide 3-kinase inhibitors
- Pyridines
- Pyrrolidines
- Thiazoles
- Trifluoromethyl compounds
- Ureas
- RTT