Abemaciclib
 | |
| Names | |
|---|---|
| Pronunciation | /ʌˌbɛməˈsaɪklɪb/ u-BEM-ə-SY-klib |
| Trade names | Verzenio, Verzenios, Ramiven, Zenlistik, others |
| Other names | LY2835219 |
| |
| Clinical data | |
| Drug class | CDK inhibitor[1] |
| Main uses | Breast cancer[1] |
| Side effects | Diarrhea, low white blood cells, nausea, infections, tiredness, hair loss, low platelets[2] |
| Routes of use | By mouth (tablets) |
| External links | |
| AHFS/Drugs.com | Monograph |
| Legal | |
| License data |
|
| Legal status |
|
| Pharmacokinetics | |
| Bioavailability | 45% |
| Protein binding | 96.3% |
| Elimination half-life | 18.3 hrs |
| Excretion | 81% via feces, 3% via urine |
| Chemical and physical data | |
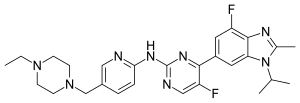
| Formula | C27H32F2N8 |
| Molar mass | 506.606 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Abemaciclib, sold under the brand names Verzenio among others, is a medication used to treat breast cancer.[1] Specifically it is used for advanced cases that are HR positive but HER2 negative.[1] It is taken by mouth.[3]
Common side effects include diarrhea, low white blood cells, nausea, infections, tiredness, hair loss, and low platelets.[2] Other side effects may include pneumonitis, liver problems, and blood clots.[2] Use in pregnancy may harm the baby.[2] It is a CDK inhibitor which blocks the activity of CDK4 and CDK6.[1]
Abemaciclib was approved for medical use in the United States in 2017 and Europe in 2018.[2][1] In the United Kingdom 4 weeks costs the NHS about £2,950 as of 2021.[3] In the United States this amount costs about 13,700 USD.[4]
Medical uses
Since September 2017 Abemaciclib is approved in the US for "adult patients who have hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative advanced or metastatic breast cancer that has progressed after taking therapy that alters a patient's hormones".[5]
In studies that compared fulvestrant plus abemaciclib to fulvestrant plus placebo in breast cancer patients, progression-free survival under abemaciclib therapy was 16.4 months on average, as compared to 9.3 months under the placebo arm.
Dosage
It is typically taken at a dose of 150 mg twice per day.[3]
Side effects
Side effects that occurred in 20% or more of patients in studies were diarrhea, nausea and vomiting, leukopenia (low white blood cell count) including neutropenia, anemia (low red blood cell count), thrombocytopenia (low platelet count), stomach pain, infections, fatigue, decreased appetite, and headache.[6][7]
Interactions
As abemaciclib is mainly metabolized by the liver enzyme CYP3A4, inhibitors of this enzyme (such as ketoconazole) are expected to increase its blood plasma concentrations. Conversely, CYP3A4 inducers lower plasma concentrations of abemaciclib, as has been shown in a study with rifampicin.[7]
Pharmacology
Mechanism of action
Like the related drugs palbociclib and ribociclib, abemaciclib inhibits the enzymes cyclin-dependent kinase 4 (CDK4) and cyclin-dependent kinase 6 (CDK6).[7] These enzymes are responsible for phosphorylating and thus deactivating the retinoblastoma protein, which plays a role in cell cycle progression from the G1 (first gap) to the S (synthesis) phase.[8] Blocking this pathway prevents cells from progressing to the S phase, thereby inducing apoptosis (cell death).[7] In vitro analysis using cancer cell lines, it is reported that abemaciclib induces non‐apoptotic cell death characterized by formation of cytoplasmic vacuoles derived from lysosomes. This result suggest that there may be a mechanism of action other than inhibition of a cyclin-dependent kinase.[9]
Pharmacokinetics

After oral intake, absolute bioavailability is 45%. Highest blood plasma concentrations are reached after 8 hours on average (range: 4.1–24.0 hours). When in the circulation, 96.3% of abemaciclib is bound to plasma proteins. The substance is mainly metabolized by the liver enzyme CYP3A4 to N-desethylabemaciclib (M2), and to a lesser extent to hydroxy derivatives (M18, M20) and another oxidative metabolite (M1). These metabolites have high plasma protein binding rates similar to the parent substance.[7]
Abemaciclib is excreted mainly via the feces (81%) and to a small extent via the urine (3%). Its elimination half-life is 18.3 hours on average.[7]
Chemistry
Abemaciclib may be synthesized in a four step manner using a Suzuki coupling, followed by a Buchwald–Hartwig amination with the final step being a reductive amination using the Leuckart reaction.[10]
Research
As of early 2016, abemaciclib was involved in 3 Phase III clinical trials:
- The JUNIPER Study[11] is comparing abemaciclib against erlotinib in patients with stage IV non-small-cell lung carcinoma[12] Due to collect data until September 2017.[11]
- The MONARCH 2 study is investigating the effectiveness of abemaciclib in combination with fulvestrant for women with breast cancer.[13] It is due to end in Feb 2017.[14] In March 2017, Eli Lilly announced that it had met its primary endpoint of superior progression-free survival (PFS) over placebo plus fulvestrant in patients with estrogen receptor positive and HER2 negative advanced or metastatic breast cancer.[15] This result led to the September 2017 FDA approval.[16]
- The MONARCH 3 study[17] is investigating the effectiveness of abemaciclib, plus either anastrozole or letrozole, as a first-line treatment for women with breast cancer.[18] The trial is expected to end in June 2017.[17]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 "Verzenios". Archived from the original on 3 June 2021. Retrieved 13 January 2022.
- ↑ 2.0 2.1 2.2 2.3 2.4 "DailyMed - VERZENIO- abemaciclib tablet". dailymed.nlm.nih.gov. Archived from the original on 13 May 2021. Retrieved 13 January 2022.
- ↑ 3.0 3.1 3.2 BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 1010. ISBN 978-0857114105.
- ↑ "Verzenio Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 16 January 2021. Retrieved 13 January 2022.
- ↑ "FDA approves new treatment for certain advanced or metastatic breast cancers" (Press release). Food and Drug Administration. 28 September 2017. Archived from the original on 23 April 2019. Retrieved 24 August 2021.
- ↑ Drugs.com: Abemaciclib Monograph. Accessed 2017-11-22.
- ↑ 7.0 7.1 7.2 7.3 7.4 7.5 "Highlights of Prescribing Information for Verzenio" (PDF). September 2017. Archived (PDF) from the original on 5 September 2021. Retrieved 24 August 2021.
- ↑ Goodrich DW, Wang NP, Qian YW, Lee EY, Lee WH (October 1991). "The retinoblastoma gene product regulates progression through the G1 phase of the cell cycle". Cell. 67 (2): 293–302. doi:10.1016/0092-8674(91)90181-w. PMID 1655277. S2CID 12990398.
- ↑ Hino H, Iriyama N, Kokuba H, et al. (June 2020). "Abemaciclib induces atypical cell death in cancer cells characterized by formation of cytoplasmic vacuoles derived from lysosomes". Cancer Sci. 111 (6): 2132–2145. doi:10.1111/cas.14419. PMC 7293084. PMID 32304130.
- ↑ Frederick MO, Kjell DP (February 2015). "A synthesis of abemaciclib utilizing a Leuckart–Wallach reaction". Tetrahedron Letters. 56 (7): 949–951. doi:10.1016/j.tetlet.2014.12.082.
- ↑ 11.0 11.1 "A Study of Abemaciclib (LY2835219) in Participants With Previously Treated KRAS Mutated Lung Cancer (JUNIPER)". Archived from the original on 4 June 2021. Retrieved 24 August 2021.
- ↑ Goldman JW, Shi P, Reck M, Paz-Ares L, Koustenis A, Hurt KC (January 2016). "Treatment Rationale and Study Design for the JUNIPER Study: A Randomized Phase III Study of Abemaciclib With Best Supportive Care Versus Erlotinib With Best Supportive Care in Patients With Stage IV Non-Small-Cell Lung Cancer With a Detectable KRAS Mutation Whose Disease Has Progressed After Platinum-Based Chemotherapy". Clinical Lung Cancer. 17 (1): 80–4. doi:10.1016/j.cllc.2015.08.003. PMID 26432508.
- ↑ Llombart A, Toi M, Klise SR, Frenzel M, Chan EM, Sledge GW (30 April 2015). "Abstract OT1-1-07: A phase III study of abemaciclib (LY2835219) combined with fulvestrant in women with hormone receptor positive (HR+), human epidermal growth factor receptor 2 negative (HER2-) breast cancer (MONARCH 2)". Cancer Research. 75 (9 Supplement): OT1–1–07–OT1–1–07. doi:10.1158/1538-7445.SABCS14-OT1-1-07.
- ↑ "A Study of Abemaciclib (LY2835219) Combined With Fulvestrant in Women With Hormone Receptor Positive HER2 Negative Breast Cancer (MONARCH 2)". Archived from the original on 3 June 2021. Retrieved 24 August 2021.
- ↑ "Lilly Announces Phase 3 MONARCH 2 Breast Cancer Study of Abemaciclib Met Primary Endpoint of Progression-Free Survival, Eli Lilly, 20 March 2017". Archived from the original on 1 December 2017. Retrieved 24 August 2021.
- ↑ "Abemaciclib Receives FDA Approval for Certain Metastatic Breast Cancers. Sept 2017". Archived from the original on 19 December 2018. Retrieved 24 August 2021.
- ↑ 17.0 17.1 "A Study of Nonsteroidal Aromatase Inhibitors Plus Abemaciclib (LY2835219) in Postmenopausal Women With Breast Cancer (MONARCH 3)". Archived from the original on 4 June 2021. Retrieved 24 August 2021.
- ↑ Goetz MP, Toi M, Klise S, Frenzel M, Bourayou N, Di Leo A (2015). "MONARCH 3: A randomized phase III study of anastrozole or letrozole plus abemaciclib, a CDK4/6 inhibitor, or placebo in first-line treatment of women with HR+, HER2-locoregionally recurrent or metastatic breast cancer (MBC)". Journal of Clinical Oncology. 33 (15_suppl): TPS624. doi:10.1200/jco.2015.33.15_suppl.tps624. Archived from the original on 7 April 2016. Retrieved 30 March 2016.
External links
| External sites: | |
|---|---|
| Identifiers: |
|
- Pages using duplicate arguments in template calls
- Use dmy dates from November 2019
- Articles with invalid date parameter in template
- Drugs with non-standard legal status
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without UNII source
- Drugs missing an ATC code
- Articles containing unverified chemical infoboxes
- Aminopyrimidines
- Benzimidazoles
- Breakthrough therapy
- Eli Lilly and Company brands
- Fluoroarenes
- Isopropyl compounds
- Piperazines
- Protein kinase inhibitors
- Pyridines
- RTT