Guanine
| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
2-Amino-1,9-dihydro-6H-purin-6-one | |||
| Other names
2-amino-6-hydroxypurine,
2-aminohypoxanthine, Guanine | |||
| Identifiers | |||
3D model (JSmol)
|
| ||
| 147911 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.000.727 | ||
| EC Number |
| ||
| 431879 | |||
| KEGG | |||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C5H5N5O | |||
| Molar mass | 151.13 g/mol | ||
| Appearance | White amorphous solid. | ||
| Density | 2.200 g/cm3 (calculated) | ||
| Melting point | 360 °C (680 °F; 633 K) decomposes | ||
| Boiling point | Sublimes | ||
| Insoluble. | |||
| Acidity (pKa) | 3.3 (amide), 9.2 (secondary), 12.3 (primary)[1] | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
Irritant | ||
| NFPA 704 (fire diamond) | |||
| Flash point | Non-flammable | ||
| Related compounds | |||
Related compounds
|
Cytosine; Adenine; Thymine; Uracil | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Guanine (/ˈɡwɑːnɪn/ ⓘ) (symbol G or Gua) is one of the four main nucleobases found in the nucleic acids DNA and RNA, the others being adenine, cytosine, and thymine (uracil in RNA). In DNA, guanine is paired with cytosine. The guanine nucleoside is called guanosine.
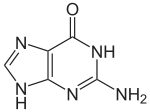
With the formula C5H5N5O, guanine is a derivative of purine, consisting of a fused pyrimidine-imidazole ring system with conjugated double bonds. This unsaturated arrangement means the bicyclic molecule is planar.
Properties
Guanine, along with adenine and cytosine, is present in both DNA and RNA, whereas thymine is usually seen only in DNA, and uracil only in RNA. Guanine has two tautomeric forms, the major keto form (see figures) and rare enol form.[citation needed]
It binds to cytosine through three hydrogen bonds. In cytosine, the amino group acts as the hydrogen bond donor and the C-2 carbonyl and the N-3 amine as the hydrogen-bond acceptors. Guanine has the C-6 carbonyl group that acts as the hydrogen bond acceptor, while a group at N-1 and the amino group at C-2 act as the hydrogen bond donors.[citation needed]
Guanine can be hydrolyzed with strong acid to glycine, ammonia, carbon dioxide, and carbon monoxide. First, guanine gets deaminated to become xanthine.[2] Guanine oxidizes more readily than adenine, the other purine-derivative base in DNA. Its high melting point of 350 °C reflects the intermolecular hydrogen bonding between the oxo and amino groups in the molecules in the crystal. Because of this intermolecular bonding, guanine is relatively insoluble in water, but it is soluble in dilute acids and bases.
History
The first isolation of guanine was reported in 1844 by the German chemist Julius Bodo Unger (1819–1885), who obtained it as a mineral formed from the excreta of sea birds, which is known as guano and which was used as a source of fertilizer; guanine was named in 1846.[3] Between 1882 and 1906, Emil Fischer determined the structure and also showed that uric acid can be converted to guanine.[4]
Synthesis
Trace amounts of guanine form by the polymerization of ammonium cyanide (NH
4CN). Two experiments conducted by Levy et al. showed that heating 10 mol·L−1 NH
4CN at 80 °C for 24 hours gave a yield of 0.0007%, while using 0.1 mol·L−1 NH
4CN frozen at −20 °C for 25 years gave a 0.0035% yield. These results indicate guanine could arise in frozen regions of the primitive earth. In 1984, Yuasa reported a 0.00017% yield of guanine after the electrical discharge of NH
3, CH
4, C
2H
6, and 50 mL of water, followed by a subsequent acid hydrolysis. However, it is unknown whether the presence of guanine was not simply a resultant contaminant of the reaction.[5]
- 10NH3 + 2CH4 + 4C2H6 + 2H2O → 2C5H8N5O (guanine) + 25H2
A Fischer–Tropsch synthesis can also be used to form guanine, along with adenine, uracil, and thymine. Heating an equimolar gas mixture of CO, H2, and NH3 to 700 °C for 15 to 24 minutes, followed by quick cooling and then sustained reheating to 100 to 200 °C for 16 to 44 hours with an alumina catalyst, yielded guanine and uracil:
- 10CO + H2 + 10NH3 → 2C5H8N5O (guanine) + 8H2O
Another possible abiotic route was explored by quenching a 90% N2–10%CO–H2O gas mixture high-temperature plasma.[6]
Traube's synthesis involves heating 2,4,5-triamino-1,6-dihydro-6-oxypyrimidine (as the sulfate) with formic acid for several hours.

Biosynthesis
Guanine is not synthesized de novo[clarification needed], instead it's split from the more complex molecule, guanosine, by the enzyme guanosine phosphorylase:
- guanosine + phosphate guanine + alpha-D-ribose 1-phosphate
Guanine can be synthesized de novo, with the rate-limiting enzyme of inosine monophosphate dehydrogenase.
Other occurrences and biological uses
The word guanine derives from the Spanish loanword guano ("bird/bat droppings"), which itself is from the Quechua word wanu, meaning "dung". As the Oxford English Dictionary notes, guanine is "A white amorphous substance obtained abundantly from guano, forming a constituent of the excrement of birds".[7]
In 1656 in Paris, a Mr. Jaquin extracted from the scales of the fish Alburnus alburnus so-called "pearl essence",[8] which is crystalline guanine.[9] In the cosmetics industry, crystalline guanine is used as an additive to various products (e.g., shampoos), where it provides a pearly iridescent effect. It is also used in metallic paints and simulated pearls and plastics. It provides shimmering luster to eye shadow and nail polish. Facial treatments using the droppings, or guano, from Japanese nightingales have been used in Japan and elsewhere, because the guanine in the droppings makes the skin look paler.[10] Guanine crystals are rhombic platelets composed of multiple transparent layers, but they have a high index of refraction that partially reflects and transmits light from layer to layer, thus producing a pearly luster. It can be applied by spray, painting, or dipping. It may irritate the eyes. Its alternatives are mica, faux pearl (from ground shells),[11] and aluminium and bronze particles.
Guanine has a very wide variety of biological uses that include a range of functions ranging in both complexity and versatility. These include camouflage, display, and vision among other purposes.[12]
Spiders, scorpions, and some amphibians convert ammonia, as a product of protein metabolism in the cells, to guanine, as it can be excreted with minimal water loss.[12]
Guanine is also found in specialized skin cells of fish called iridocytes (e.g., the sturgeon),[13][12] as well as being present in the reflective deposits of the eyes of deep-sea fish and some reptiles, such as crocodiles and chameleons.[13]
On 8 August 2011, a report, based on NASA studies with meteorites found on Earth, was published suggesting building blocks of DNA and RNA (guanine, adenine and related organic molecules) may have been formed extra-terrestrially in outer space.[14][15][16]
See also
References
- ^ Dawson, R.M.C., et al., Data for Biochemical Research, Oxford, Clarendon Press, 1959.
- ^ Angstadt. "Purines and pyrimidines". Retrieved 2008-03-27.
{{cite journal}}: Cite journal requires|journal=(help) - ^ Guanine was first isolated in 1844 by Julius Bodo Unger (1819–1885), a student of Heinrich Gustav Magnus. See:
- Paul O. P. Ts'o, Basic Principles in Nucleic Acid Chemistry, vol. 1 (New York, New York: Academic Press, 1974), page 7.
- Magnus (1844) "Ueber das Vorkommen von Xanthicoxyd im Guano" (On the occurrence of xanthic oxide in guano), Annalen der Chemie und Pharmacie, 51 : 395-397.
- B. Unger (1846) "Bemerkungen zu obiger Notiz" (Comments on the above notice), Annalen der Chemie und Pharmacie, 58 : 18-20. From page 20: " ... desshalb möchte ich den Namen Guanin vorschlagen, welcher an seine Herkunft erinnert." ( ... therefore I would like to suggest the name guanine, which is reminiscent of its origin.)
- B. Unger (1846) "Das Guanin und seine Verbindungen" (Guanine and its compounds), Annalen der Chemie und Pharmacie, 59 : 58-68.
- ^ "Emil Fischer - Biographical".
- ^ Levy, Matthew; Stanley L. Miller; John Oró (August 1999). "Production of Guanine from NH4CN Polymerizations". Journal of Molecular Evolution. 49 (2): 165–8. Bibcode:1999JMolE..49..165L. doi:10.1007/PL00006539. PMID 10441668. S2CID 32194418. - quotes the Yuasa paper and cites the possibility of there being a contaminant in the reaction.
- ^ Miyakawa, S; Murasawa, K.; Kobayashi, K.; Sawaoka, AB. (December 2000). "Abiotic synthesis of guanine with high-temperature plasma". Orig Life Evol Biosph. 30 (6): 557–66. Bibcode:2000OLEB...30..557M. doi:10.1023/A:1026587607264. PMID 11196576. S2CID 25417484.
- ^ OED. "guanine" and also "guano".
- ^ Johann Rudolf von Wagner, Ferdinand Fischer, and L. Gautier, Traité de chimie industrielle (Treatise on industrial chemistry), 4th ed., (Paris, France: Masson & Co., 1903), vol. 2, pp. 64–65.
- ^ In 1861 the French chemist Charles-Louis Barreswil (1817–1870) found that "pearl essence" was guanine. See: Barreswil (1861) "Sur le blanc d'ablette qui sert à la fabrication des perles fausses" (On the white of ablette that's used in making imitation pearls), Comptes rendus, 53 : 246.
- ^ Whitworth, Melissa (2008-10-16). "Geisha facial, the 'latest beauty secret' of Victoria Beckham, brought to the masses". Lifestyle. Telegraph. Archived from the original on 2008-12-05. Retrieved 2008-11-20.
- ^ "How Pearls are Made...Faux, Fake, Imitation, Simulated or Man-made".
{{cite journal}}: Cite journal requires|journal=(help) - ^ a b c Gur, Dvir; Palmer, Benjamin A.; Weiner, Steve; Addadi, Lia (2017). "Light manipulation by guanine crystals in organisms: biogenic scatterers, mirrors, multilayer reflectors and photonic crystals". Advanced Functional Materials. 27 (6): 1603514. doi:10.1002/adfm.201603514. S2CID 136383728.
- ^ a b Fox, D.L. (1979). Biochromy, natural coloration of living things. University of California Press. ISBN 978-0-520-03699-4.
- ^ Callahan; Smith, K.E.; Cleaves, H.J.; Ruzica, J.; Stern, J.C.; Glavin, D.P.; House, C.H.; Dworkin, J.P. (11 August 2011). "Carbonaceous meteorites contain a wide range of extraterrestrial nucleobases". Proc. Natl. Acad. Sci. U.S.A. 108 (34). PNAS: 13995–8. Bibcode:2011PNAS..10813995C. doi:10.1073/pnas.1106493108. PMC 3161613. PMID 21836052.
- ^ Steigerwald, John (8 August 2011). "NASA Researchers: DNA Building Blocks Can Be Made in Space". NASA. Retrieved 2011-08-10.
- ^ ScienceDaily Staff (9 August 2011). "DNA Building Blocks Can Be Made in Space, NASA Evidence Suggests". ScienceDaily. Retrieved 2011-08-09.
External links
- Guanine MS Spectrum
- Guanine at chemicalland21.com
- Pages using the Phonos extension
- CS1 errors: missing periodical
- Articles with short description
- Short description is different from Wikidata
- Chemical articles with multiple compound IDs
- Multiple chemicals in an infobox that need indexing
- ECHA InfoCard ID from Wikidata
- Articles containing unverified chemical infoboxes
- Chembox image size set
- Short description matches Wikidata
- Pages including recorded pronunciations
- All articles with unsourced statements
- Articles with unsourced statements from February 2024
- Pages using multiple image with auto scaled images
- Wikipedia articles needing clarification from March 2022
- Commons category link is on Wikidata
- Articles with BNF identifiers
- Articles with BNFdata identifiers
- Nucleobases
- Purines
- Cosmetics chemicals
- Organic minerals





