Cladribine
 | |
| Names | |
|---|---|
| Trade names | Leustatin, Mavenclad, others[1] |
| Other names | 2-chlorodeoxyadenosine |
| |
| Clinical data | |
| Drug class | Antimetabolite (purine analogue)[2] |
| Main uses | Hairy cell leukemia, chronic lymphocytic leukemia, non-Hodgkin lymphoma, cutaneous T-cell lymphoma, relapsing-remitting multiple sclerosis[3][4] |
| Side effects | Infection, anxiety, hair loss, arrythmias, diarrhea, fever, muscle pain, rash, bleeding[4] |
| Pregnancy category |
|
| Routes of use | Intravenous, subcutaneous (liquid), by mouth (tablet) |
| External links | |
| AHFS/Drugs.com | Injectable: Monograph By mouth: Monograph |
| MedlinePlus | a693015 |
| Legal | |
| License data |
|
| Legal status | |
| Pharmacokinetics | |
| Bioavailability | 100% (i.v.); 37 to 51% (by mouth)[5] |
| Protein binding | 25% (range 5-50%);[6] up to 20% (by mouth) [7] |
| Metabolism | Mostly via intracellular kinases; 15-18% is excreted unchanged[6]
IV and SQ bolus: 15-18% is excreted unchanged By mouth, 25% (±21%) of dose is excreted unchanged in urine and 3.8% as a metabolite[7] |
| Elimination half-life | ~10 hours IV and SQ[6] and 18.4 to 19.7 hours after by mouth |
| Excretion | Urinary[6] |
| Chemical and physical data | |
| Formula | C10H12ClN5O3 |
| Molar mass | 285.69 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Cladribine, sold under the brand name Leustatin among others, is a medication used to treat hairy cell leukemia, chronic lymphocytic leukemia, non-Hodgkin lymphoma, and cutaneous T-cell lymphoma.[3] It is also used for relapsing-remitting multiple sclerosis.[4] It is taken by mouth or by injection into a vein or under the skin.[4]
Common side effects include infection, anxiety, hair loss, arrythmias, diarrhea, fever, muscle pain, rash, and bleeding.[4] Other side effects may include bone marrow suppression, progressive multifocal encephalopathy (PML), tumor lysis syndrome, and nerve damage.[4] Use in pregnancy may harm the baby.[4] It is an antimetabolite, specifically a purine analogue, which interferes with the production of new DNA by lymphocytes.[2]
Cladribine has been in medical use since the 1980s, with use in parts of Europe since 1993.[2] It was also approved for medical use in the United States in 1993.[3] It is on the World Health Organization's List of Essential Medicines.[8] Some formulations are available as a generic medication.[3] In the United Kingdom 10 mg for injection costs about £160 while a 10 mg pill costs £2,050 as of 2021.[4] In the United States this amount costs about 370 USD and 9,000 USD respectively.[9][10]
Medical uses
Cancer
Cladribine is used as a first- and second-line treatment for symptomatic HCL and for B-cell chronic lymphocytic leukaemia, and is administered by intravenous or subcutaneous infusion.[11][12] Some have used the injectable formulation by mouth to treat HCL. Notably, about 37–51% of cladribine is bioavailable by mouth.[13] It is used, often in combination with other cytotoxic agents, to treat various kinds of histiocytosis, including Erdheim–Chester disease[14] and Langerhans cell histiocytosis.[15]
Multiple sclerosis
Following EMA approval of cladribine tablets for the treatment of adults with highly active RRMS in 2017, as of July 2020, Cladribine tablets have gained marketing authorisation in over 75 countries.[16] In 2019, cladribine tablets were approved by the FDA for the treatment of relapsing forms of multiple sclerosis, to include relapsing-remitting disease and active secondary progressive (SPMS) disease, in adults who have had an inadequate response to, or are unable to tolerate, an alternate drug indicated for the treatment of MS.[16][17]
As per the EU label, cladribine tablets are indicated for the treatment of adults with highly active relapsing MS as defined by clinical or imaging features: (i) patients with a relapse in the previous year and at least one T1 Gd+ lesion or 9 or more T2 lesions, while on another disease-modifying therapies or (ii) patients with two or more relapses in the previous year, whether on disease-modifying treatment or not.[18]
Two main approaches to MS treatment maintenance therapy are used – immunomodulation and immunosuppression and alternatively, immune reconstitution therapy (IRT). Classified as an IRT, Cladribine tablets are administered intermittently as a short treatment course without continuous immunosuppression. In contrast to maintenance therapies, clinical efficacy extends beyond the dosing period.[19][20][21]
Cladribine tablets are administered as 2 courses separated by 1 year (a maximum of 20 days of treatment). The recommended cumulative dose is 3.5 mg/kg body weight over 2 years, administered as 1 treatment course of 1.75 mg/kg per year. Each treatment course consists of 2 treatment weeks, one at the beginning of the first month and one at the beginning of the second month of the respective treatment year. Each treatment week consists of 4 or 5 days on which a patient receives 10mg or 20mg (1 or 2 tablets) as a single daily dose based on body weight.[18]
Before initiating treatment with cladribine tablets, blood tests, MRI and infection screening must be performed. Due to an increased risk of herpes zoster with Cladribine tablets, patients who are antibody-negative for varicella zoster virus are recommended to be vaccinated before starting treatment. Treatment should not be initiated within 4 to 6 weeks of receiving a live or attenuated live vaccine because of a risk of active infection. Vaccination with live or attenuated live vaccines should also be avoided during and after treatment, but can be considered when lymphocyte counts have recovered to ≥1000 cells/mm3.[18]
Following completion of the two treatment courses, no further treatment or additional monitoring is required.[18]
Side effects
Hairy cell leukemia
Injectable cladribine suppresses the body's ability to make new lymphocytes, natural killer cells, and neutrophils (called myelosuppression); data from HCL studies showed that about 70% of people taking the drug had fewer white blood cells and about 30% developed infections and some of those progressed to septic shock; about 40% of people taking the drug had fewer red blood cells and became severely anaemic; and about 10% of people had too few platelets.[12] At the dosage used to treat HCL in two clinical trials, 16% of people had rashes and 22% had nausea, the nausea generally did not lead to vomiting.[12]
Multiple sclerosis
Cladribine tablets target the cells of the adaptive immune system with minimal impact on innate immune cells. Although the exact mechanism by which cladribine exerts its therapeutic effect is not fully elucidated, it is proposed to have a transient effect on B and T lymphocyte depletion, interrupting the cascade of immune events central to MS. As a result, a reduction in lymphocyte count (lymphopenia) may be reported following treatment.[18] In clinical trials, lymphocyte levels above Grade 0 (≥1000 cells/mm3) and Grade 1 (<1000–800 cells/mm3) were maintained in most patients, with levels continuing to improve after the 2-year dosing period.[22] Less than 1% of patients developed Grade 4 lymphopenia (<200 cells/mm3). It is important that patients with lymphocyte counts below 500 cells/mm3 should be actively monitored for signs suggestive of infection and that anti-infective treatments are given to at-risk patients.[18][23]
Despite the initial reduction in lymphocyte counts following treatment, studies showed the overall risk of infection in patients receiving Cladribine tablets was comparable to those who received placebo, except for herpes zoster infection.[23] Due to this increased risk, it is recommended that patients are screened for varicella zoster virus and antibody-negative patients are vaccinated prior to receiving treatment.[18] In an analysis of post-approval data, as of 2020, no new infection safety signals were observed in over 18,000 patients.[24]
Progressive multifocal leukoencephalopathy (PML) has been reported in patients with HCL treated with parenteral cladribine.[18] However, in up to 10 years of follow-up of patients receiving Cladribine tablets for MS, no cases of PML have been observed; baseline MRI must be performed prior to initiating treatment.[18]
In clinical trials, malignancies were observed more frequently in patients treated with Cladribine tablets compared with patients who received placebo. Compared with a matched reference population from the Global Cancer Observatory database, Cladribine tablets had no increased risk of malignancy in long-term real-world evidence data.[18][23]
Pregnancy
Cladribine may cause harm to the baby if used during pregnancy and is listed by the FDA as pregnancy category D; safety and efficacy in children has not been established.[12]
The use of cladribine tablets is contraindicated in pregnant women, and women of childbearing potential must use effective contraception to prevent pregnancy during treatment and 6 months after receiving the last dose.[18]
Mechanism of action
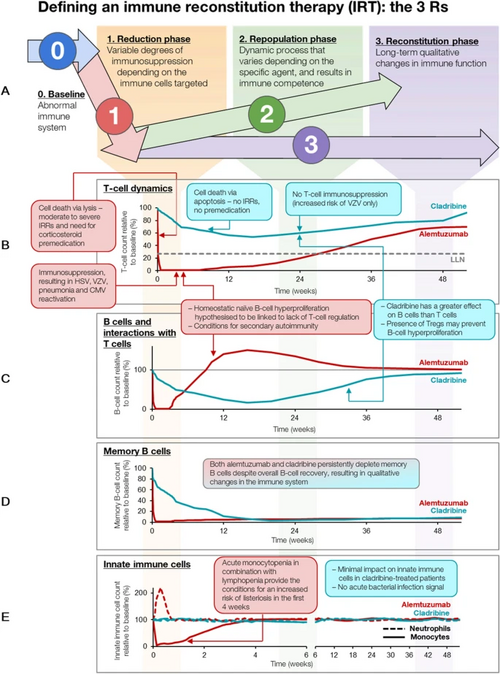
Cladribine (2-chloro-2'-deoxyadenosine [2-CdA]) is a purine analogue that selectively targets and suppresses lymphocytes implicated in the underlying pathogenesis of multiple sclerosis (MS) and B-cell leukaemia.[26][7][27] Chemically, it mimics the nucleoside adenosine. However, unlike adenosine, it is relatively resistant to breakdown by the enzyme adenosine deaminase (ADA), which causes it to accumulate in targeted cells and interfere with the cell's ability to process DNA.[7] Cladribine is taken up by cells via transporter proteins. Once inside a cell, cladribine undergoes phosphorylation by the enzyme deoxycytidine kinase (DCK) to produce mononucleotide 2-chlorodeoxyadenosine 5’monophosphate (2-CdAMP), which is subsequently phosphorylated to the triphosphorylated active compound 2-chlorodeoxyadenosine 5’triphosphate (2-CdATP). Activated cladribine is incorporated into cellular DNA, which triggers apoptosis. Accumulation of cladribine into cells is dependent on the ratio of 2-CdATP and 5'-nucleotidase (5’-NT), which breaks down and inactivates the compound. This ratio differs between cell types, with high levels in T and B lymphocytes, resulting in selective targeting of these cells. In contrast, CdATP:5'NT is relatively low in other cell types, thus sparing numerous non-haematological cells.[26][7]
As a purine analogue, cladribine (2-chloro-2'-deoxyadenosine [2-CdA]) is taken up into rapidly proliferating cells, including B and T lymphocytes, to be incorporated into DNA synthesis. Chemically, it mimics nucleoside adenosine; however, unlike adenosine, cladribine has a chlorine molecule at position 2, which renders it partially resistant to breakdown by ADA. This causes it to accumulate in cells and interfere with the targeted cell's ability to process DNA.[7][26]
Cladribine is taken up by specific nucleoside transporter proteins. Once inside a cell, cladribine undergoes phosphorylation by the enzyme deoxycytidine kinase (DCK) to produce mononucleotide 2-chlorodeoxyadenosine 5’monophosphate (2-CdAMP), which is subsequently phosphorylated to the triphosphorylated active compound, 2-chlorodeoxyadenosine 5’triphosphate (2-CdATP).[26][7]
Activated cladribine is incorporated into the DNA synthesis pathway, where it disrupts DNA repair and synthesis, resulting in an accumulation of DNA strand breaks[26][7][28] This is followed by the activation of transcription factor p53, the release of cytochrome c from mitochondria and eventual programmed cell death (apoptosis).[28] This process occurs over approximately 2 months, with a peak level of cell depletion 4–8 weeks after treatment.[29]
Another family of enzymes, the 5'-nucleotidase (5'-NT) family, is also capable of dephosphorylating cladribine, making it inactive. The most important subtypes of this group appear to be cytosolic 5'-NT, c-5NCT1A and c-NT1B, which are cytosolically active and specific for purine analogues.[30]
Accumulation of cladribine into cells is dependent on the ratio of 2-CdATP and 5'-NT. This ratio differs between cell types, with high levels in T and B lymphocytes, making them particularly susceptible to cell death. The cells with the highest ratios are B cells, especially germinal centre and naïve B cells. This helps to explain which B cells are more vulnerable to cladribine-mediated apoptosis. DCK is the rate-limiting enzyme for conversion of the cladribine prodrug into its active triphosphate form, leading to the selective depletion of dividing and non-dividing T and B lymphocytes. In contrast, the CdATP:5'-NT ratio is relatively low in other cell types, thus sparing numerous non-hematologic cells.[26][7][30]
In MS, cladribine's effectiveness may be due to its ability to effectively deplete B cells, in particular memory B cells.[31] In the pivotal phase 3 clinical trial of oral cladribine in MS, CLARITY, cladribine selectively depleted 80% of peripheral B cells, compared to only 40–45% of CD4+ T cells and 15‒30% CD8+ T cells.[32] More recently, cladribine has been shown to induce long term, selective suppression of certain subtypes of B cells, especially memory B cells.[30]
Although cladribine is selective for B cells, the long-term suppression of memory B cells, which may contribute to its effect in MS, is not explained by gene or protein expression. Instead, cladribine appears to deplete the entire B cell department, but while naïve B cells rapidly move from lymphoid organs, the memory B cell pool repopulates slowly from the bone marrow. Both HCL and B-cell chronic lymphocytic leukaemia are types of B cell blood cancers.
History
Hairy cell leukemia
Ernest Beutler and Dennis A. Carson had studied adenosine deaminase deficiency and recognised that because the lack of adenosine deaminase led to the destruction of B cell lymphocytes, a drug designed to inhibit adenosine deaminase might be useful in lymphomas. Carson then synthesised cladribine, and through clinical research at Scripps starting in the 1980s, Beutler tested it as intravenous infusion and found it was especially useful to treat HCL. No pharmaceutical companies were interested in selling the drug because HCL was an orphan disease, so Beutler's lab synthesised and packaged it and supplied it to the hospital pharmacy; the laboratory also developed a test to monitor blood levels. This was the first treatment that led to prolonged remission of HCL, which was previously untreatable.[33]: 14–15
In February 1991, Scripps began a collaboration with Johnson & Johnson (J&J) to bring intravenous cladribine to market, and by December of that year, J&J had filed a new drug application (NDA); cladribine was approved by the FDA in 1993 for HCL as an orphan drug,[34] and was approved in Europe later that year.[35]: 2
The subcutaneous formulation was developed in Switzerland in the early 1990s and it was commercialised by Lipomed GmbH in the 2000s.[35]: 2 [36]
Multiple sclerosis
In the mid-1990s, Beutler, in collaboration with Jack Sipe, a neurologist at Scripps Institute, ran several clinical trials exploring the utility of cladribine in multiple sclerosis, based on the drug's immunosuppressive effects. Sipe's insight into MS, and Beutler's interest in MS due to his sister having the disease, initiated a very productive collaboration.[37] Ortho-Clinical, a subsidiary of J&J, filed an NDA for cladribine for MS in 1997 but withdrew it in the late 1990s after discussion with the FDA proved that more clinical data would be needed.[37]
Ivax acquired the rights for oral administration of cladribine to treat MS from Scripps in 2000,[38] and partnered with Serono in 2002.[39] Ivax was acquired by Teva in 2006,[40][41] and Merck KGaA acquired control of Serono's drug business in 2006.[42]
An oral formulation of the drug with cyclodextrin was developed[43]: 16 by Ivax and Serono, and then Merck KGaA conducted clinical trials. Merck KGaA submitted an application to the European Medicines Agency in 2009, which was rejected in 2010, and an appeal was denied in 2011.[43]: 4–5 Likewise Merck KGaA's NDA with the FDA rejected in 2011.[44]
The ratio of benefit to harm was not clear to regulators, and further studies were requested to address concerns related to severe lymphopenia and cancer cases observed during pivotal trials.[43]: 54–55 Phase II and III MS clinical trials were still ongoing at the time of the rejections, and Merck KGaA committed to completing them.[44] A meta-analysis of data from clinical trials comparing the risk of cancer and other disease-modifying therapies showed that Cladribine tablets did not increase the risk of cancer at the doses used in the initial clinical trials.[45]
Based on the supporting data from the completed clinical trials that confirmed no increased risk of cancer, Merck announced it would again seek regulatory approval.[46] In 2016, the EMA accepted its application for review.[47] On June 22, 2017, the EMA's Committee for Medicinal Products for Human Use (CHMP) adopted a positive opinion, recommending the granting of a marketing authorisation for the treatment of relapsing forms of multiple sclerosis.[48]
Cladribine tablets were later approved in Europe, in August 2017, for highly active RRMS, and has since been approved by the FDA for the treatment of relapsing-remitting and secondary progressive MS in the US.[49]
Research
Cladribine has been studied as part of a multidrug chemotherapy regimen for drug-resistant T-cell prolymphocytic leukaemia.[50]
Multiple sclerosis
Clinical trial results have shown that Cladribine tablets can be an effective treatment for highly active, relapsing forms of MS, with significant clinical benefits in relapse rate, disability progression, and radiological measures.[51] Compared with placebo, patients who received Cladribine tablets (3.5 mg/kg) in the CLARITY study had a 58% reduction in annualized relapse rate[52] and 47% of patients showed no evidence of disease activity at 2 years.[53] Clinical improvements can be observed at Week 24 of treatment,[53][54][55] and benefits may be sustained up to 4 years, beyond the 2-year dosing period and recovery of total lymphocytes.[51][22][56] Post-hoc analyses of clinical trial data showed that 89% of patients remained free from disability progression two years after treatment.[57]
Further analyses of a subgroup of patients in the CLARITY study who had very active MS showed a 67% reduction in relapse rates and an 82% reduction in disability progression in those treated with Cladribine tablets. Similarly, clinical improvements were seen in lesion burden on MRI scans in this population.[58]
Studies evaluating the treatment effects of Cladribine tablets across a spectrum of baseline demographics and disease characteristics showed that the relative risk of relapse was significantly reduced compared with placebo, irrespective of previous treatment experience.[59]
Furthermore, treatment with Cladribine tablets has been shown to significantly reduce the rate of brain atrophy in patients with highly active RRMS. This reduction correlated with a reduced risk in disability progression in a retrospective analysis.[60]
In clinical trials, higher cumulative doses of Cladribine tablets did not result in further improvement in efficacy nor did additional courses after the 2-year treatment period, but was associated with a higher incidence of Grade 3 and Grade 4 lymphopenia.[18][51]
References
- ↑ "Cladribine". Drugs.com. 28 February 2020. Archived from the original on 4 March 2016. Retrieved 4 March 2020.
- ↑ 2.0 2.1 2.2 "Litak". Archived from the original on 16 April 2021. Retrieved 5 January 2022.
- ↑ 3.0 3.1 3.2 3.3 "Cladribine Monograph for Professionals". Drugs.com. Archived from the original on 5 March 2021. Retrieved 5 January 2022.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 4.7 BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 952. ISBN 978-0857114105.
- ↑ Liliemark J (February 1997). "The clinical pharmacokinetics of cladribine". Clinical Pharmacokinetics. 32 (2): 120–31. doi:10.2165/00003088-199732020-00003. PMID 9068927. S2CID 32926069.
- ↑ 6.0 6.1 6.2 6.3 "PRODUCT INFORMATION LITAK© 2 mg/mL solution for injection" (PDF). TGA eBusiness Services. St Leonards, Australia: Orphan Australia Pty. Ltd. 10 May 2010. Archived from the original on 11 September 2016. Retrieved 27 November 2014.
- ↑ 7.0 7.1 7.2 7.3 7.4 7.5 7.6 7.7 7.8 Giovannoni, G (2017). "Cladribine to Treat Relapsing Forms of Multiple Sclerosis". Neurotherapeutics. 14 (4): 874–887. doi:10.1007/s13311-017-0573-4. PMC 5722776. PMID 29168160.
- ↑ World Health Organization (2023). The selection and use of essential medicines 2023: web annex A: World Health Organization model list of essential medicines: 23rd list (2023). Geneva: World Health Organization. hdl:10665/371090. WHO/MHP/HPS/EML/2023.02.
- ↑ "Cladribine Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 20 April 2021. Retrieved 5 January 2022.
- ↑ "Mavenclad Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 22 January 2021. Retrieved 5 January 2022.
- ↑ "Leustat Injection. - Summary of Product Characteristics (SPC) - (eMC)". www.medicines.org.uk. Archived from the original on 2017-10-03. Retrieved 2021-10-27.
- ↑ 12.0 12.1 12.2 12.3 "Cladribine- cladribine injection". DailyMed. 31 December 2019. Archived from the original on 24 January 2021. Retrieved 4 March 2020.
- ↑ Liliemark, J (February 1997). "The clinical pharmacokinetics of cladribine". Clinical pharmacokinetics. 32 (2): 120–31. doi:10.2165/00003088-199732020-00003. PMID 9068927.
- ↑ "Erdheim-Chester Disease". Histiocytosis Association. Archived from the original on 6 June 2019.
- ↑ Aricò M (June 2016). "Langerhans cell histiocytosis in children: from the bench to bedside for an updated therapy". British Journal of Haematology. 173 (5): 663–70. doi:10.1111/bjh.13955. PMID 26913480.
The combination of cytarabine and cladribine is the current standard for second-line therapy of refractory cases with vital organ dysfunction.
- ↑ 16.0 16.1 Rammohan, K (2020). "The Development of Cladribine Tablets for the Treatment of Multiple Sclerosis: A Comprehensive Review". Drugs. 80 (18): 1901–1928. doi:10.1007/s40265-020-01422-9. PMC 7708385. PMID 33247831.
- ↑ Jamroz-Wiśniewska A et al. Oxid Med Cell Longev. 2020; 2020: 1654754. doi : 10.1155/2020/1654754 Archived 2021-07-16 at the Wayback Machine
- ↑ 18.00 18.01 18.02 18.03 18.04 18.05 18.06 18.07 18.08 18.09 18.10 18.11 -, MAVENCLAD® EU SmPC, February 2021. "MAVENCLAD® EU SmPC, February 2021" (PDF). MAVENCLAD® EU SmPC, February 2021. Archived (PDF) from the original on 2021-07-14. Retrieved 2021-10-27.
{{cite web}}: CS1 maint: numeric names: authors list (link) - ↑ Soelberg Sorensen P, Sellebjerg F. Ther Adv Neurol Disord 2019;12:1756286419836913. doi: 10.1177/1756286419836913 Archived 2021-07-14 at the Wayback Machine
- ↑ Giovannoni G. Curr Opin Neurol 2018;31(3):233–43 doi : 10.1097/WCO.0000000000000561 Archived 2021-07-16 at the Wayback Machine
- ↑ Baker D et al. Neurol Neuroimmunol Neuroinflamm 2017;4(4):e360 doi : 10.1212/NXI.0000000000000360 Archived 2021-07-15 at the Wayback Machine
- ↑ 22.0 22.1 Comi, G (2019). "Effect of cladribine tablets on lymphocyte reduction and repopulation dynamics in patients with relapsing multiple sclerosis". Mult Scler Relat Disord. 29: 168–74. doi:10.1016/j.msard.2019.01.038. PMID 30885375. S2CID 83461539. Archived from the original on 2021-11-02. Retrieved 2021-10-27.
- ↑ 23.0 23.1 23.2 Cook, S (2019). "Safety of cladribine tablets in the treatment of patients with multiple sclerosis: An integrated analysis". Mult Scler Relat Disord. 29: 157–67. doi:10.1016/j.msard.2018.11.021. PMID 30885374. S2CID 81873347. Archived from the original on 2021-11-02. Retrieved 2021-10-27.
- ↑ Giovannoni, Gavin (2020). "A965". Actrims-Ectrims: A965.
- ↑ Giovannoni, Gavin; Mathews, Joela (1 June 2022). "Cladribine Tablets for Relapsing–Remitting Multiple Sclerosis: A Clinician's Review". Neurology and Therapy. 11 (2): 571–595. doi:10.1007/s40120-022-00339-7. ISSN 2193-6536. Retrieved 7 December 2023.
- ↑ 26.0 26.1 26.2 26.3 26.4 26.5 Leist, Thomas P.; Weissert, Robert (2011). "Cladribine". Clinical Neuropharmacology. 34 (1): 28–35. doi:10.1097/WNF.0b013e318204cd90. PMID 21242742. S2CID 43201228. Archived from the original on 2021-11-02. Retrieved 2021-10-27.
- ↑ Jain P et al. Curr Treat Options Oncol. 2014; 15(2): 187–209. DOI: 10.1007/s11864-014-0285-5 Archived 2021-07-19 at the Wayback Machine
- ↑ 28.0 28.1 Johnston, James B (2011). "Mechanism of Action of Pentostatin and Cladribine in Hairy Cell Leukemia". Leukemia & Lymphoma. 52: 43–45. doi:10.3109/10428194.2011.570394. PMID 21463108. S2CID 207508023. Archived from the original on 2022-06-10. Retrieved 2021-10-27.
- ↑ Beutler E, Piro LD, Saven A, Kay AC, McMillan R, Longmire R, et al. (1991). "2-Chlorodeoxyadenosine (2-CdA): A Potent Chemotherapeutic and Immunosuppressive Nucleoside". Leukemia & Lymphoma. 5 (1): 1–8. doi:10.3109/10428199109068099. PMID 27463204.
- ↑ 30.0 30.1 30.2 Ceronie B, Jacobs BM, Baker D, Dubuisson N, Mao Z, Ammoscato F, et al. (May 2018). "Cladribine treatment of multiple sclerosis is associated with depletion of memory B cells". Journal of Neurology. 265 (5): 1199–1209. doi:10.1007/s00415-018-8830-y. PMC 5937883. PMID 29550884.
- ↑ Baker D, Marta M, Pryce G, Giovannoni G, Schmierer K (February 2017). "Memory B Cells are Major Targets for Effective Immunotherapy in Relapsing Multiple Sclerosis". EBioMedicine. 16: 41–50. doi:10.1016/j.ebiom.2017.01.042. PMC 5474520. PMID 28161400.
- ↑ Baker D, Herrod SS, Alvarez-Gonzalez C, Zalewski L, Albor C, Schmierer K (July 2017). "Both cladribine and alemtuzumab may effect MS via B-cell depletion". Neurology. 4 (4): e360. doi:10.1212/NXI.0000000000000360. PMC 5459792. PMID 28626781.
- ↑ Lichtman MA (2012). "Biographical Memoir: Ernest Beutler 1928–2008" (PDF). National Academy of Sciences. Archived (PDF) from the original on 2021-07-14. Retrieved 2021-10-27.
- ↑ Staff (8 March 1993). "Ortho Biotech's Leustatin For Hairy Cell Leukemia". The Pink Sheet. Archived from the original on 3 October 2017.
- ↑ 35.0 35.1 "Litak EMA package: Scientific Discussion" (PDF). Europeans Medicines Agency. 2004. Archived (PDF) from the original on 2015-09-24. Retrieved 2021-10-27.
- ↑ "Litak: Background Information on the Procedure" (PDF). Europeans Medicines Agency. 2004. Archived (PDF) from the original on 2016-08-21. Retrieved 2021-10-27.
- ↑ 37.0 37.1 Sauter, Eric; Ono, Mika. "A potential new MS treatment's long and winding road". News & Views - Scripps Research Institute. Archived from the original on 2021-05-06. Retrieved 2021-10-27.
- ↑ "Ivax to Develop Cladribine for Multiple Sclerosis". Reuters. 4 December 2000. Archived from the original on 29 August 2016. Retrieved 27 October 2021.
- ↑ Sargent C (31 October 2002). "Serono Purchases Rights To Experimental MS Drug". Dow Jones Newswires in the Wall Street Journal. Archived from the original on 24 August 2021. Retrieved 27 October 2021.
- ↑ Bayot J (26 July 2005). "Teva to Acquire Ivax, Another Maker of Generic Drugs". New York Times. Archived from the original on 8 November 2017. Retrieved 27 October 2021.
- ↑ "Teva Completes Acquisition of Ivax". Teva Press Release. 2006. Archived from the original on 2019-12-18. Retrieved 2021-10-27.
- ↑ Staff (21 September 2006). "Merck KGaA to acquire Serono". First Word Pharma. Archived from the original on 24 August 2021. Retrieved 27 October 2021.
- ↑ 43.0 43.1 43.2 "Withdrawal Assessment Report for Movectro" (PDF). Europeans Medicines Agency. 2011. Archived (PDF) from the original on 2016-08-21. Retrieved 2021-10-27.
Procedure No. EMEA/H/C/001197
- ↑ 44.0 44.1 Gever J (22 June 2011). "Merck KGaA Throws in Towel on Cladribine for MS". Archived from the original on 19 January 2021. Retrieved 27 October 2021.
- ↑ Pakpoor J, Disanto G, Altmann DR, Pavitt S, Turner BP, Marta M, et al. (December 2015). "No evidence for higher risk of cancer in patients with multiple sclerosis taking cladribine". Neurology. 2 (6): e158. doi:10.1212/nxi.0000000000000158. PMC 4592538. PMID 26468472.
- ↑ "Four years after a transatlantic slapdown, Merck KGaA will once again seek cladribine OK". Fierce Biotech. Archived from the original on 2021-07-14. Retrieved 2021-10-27.
- ↑ "Merck Receives European Medicines Agency Acceptance for Review of Marketing Authorization Application for Cladribine Tablets". PR News Wire. 18 July 2016. Archived from the original on 24 August 2021. Retrieved 27 October 2021.
- ↑ Merck. "Cladribine Tablets Receives Positive CHMP Opinion for Treatment of Relapsing Forms of Multiple Sclerosis". www.prnewswire.co.uk. Archived from the original on 2017-08-22. Retrieved 2017-08-22.
- ↑ "Cladribine approved in Europe". Merck Press Release. 25 August 2017. Archived from the original on 2 November 2021. Retrieved 27 October 2021.
- ↑ Hasanali ZS, Saroya BS, Stuart A, Shimko S, Evans J, Vinod Shah M, et al. (June 2015). "Epigenetic therapy overcomes treatment resistance in T cell prolymphocytic leukaemia". Science Translational Medicine. 7 (293): 293ra102. doi:10.1126/scitranslmed.aaa5079. PMC 4807901. PMID 26109102.
- ↑ 51.0 51.1 51.2 Giovannoni, Gavin (2018). "Safety and efficacy of cladribine tablets in patients with relapsing-remitting multiple sclerosis: Results from the randomized extension trial of the CLARITY study". Mult Scler. 24 (12): 1594–1604. doi:10.1177/1352458517727603. PMID 28870107. S2CID 1910070. Archived from the original on 2021-07-16. Retrieved 2021-10-27.
- ↑ Giovannoni, Gavin (2010). "A Placebo-Controlled Trial of Oral Cladribine for Relapsing Multiple Sclerosis". N Engl J Med. 362 (5): 416–26. doi:10.1056/NEJMoa0902533. PMID 20089960. Archived from the original on 2021-07-16. Retrieved 2021-10-27.
- ↑ 53.0 53.1 Giovannoni, Gavin (2011). "Sustained disease-activity-free status in patients with relapsing-remitting multiple sclerosis treated with cladribine tablets in the CLARITY study: a post-hoc and subgroup analysis". Lancet Neurol. 10 (4): 329–337. doi:10.1016/S1474-4422(11)70023-0. PMID 21397565. S2CID 20149620. Archived from the original on 2021-11-02. Retrieved 2021-10-27.
- ↑ Comi, G (2013). "MRI outcomes with cladribine tablets for multiple sclerosis in the CLARITY study". J Neurol. 260 (4): 1136–46. doi:10.1007/s00415-012-6775-0. PMID 23263473. S2CID 8934723. Archived from the original on 2021-07-14. Retrieved 2021-10-27.
- ↑ Schippling, Sven (2018). "CLARITY: An analysis of severity and frequency of relapses in patients with RRMS treated with Cladribine tablets or placebo". Ectrims: P549.
- ↑ Giovannoni, Gavin (2017). "Effect of cladribine tablets on relapse rates and the proportions qualified relapse-free in patients with multiple sclerosis: analysis of the CLARITY and CLARITY extension studies". EAN: P0542.
- ↑ Giovannoni, Gavin (2018). "An exploratory analysis of the efficacy of cladribine tablets 3.5mg/kg in patients with relapsing multiple sclerosis stratified according to age above and below 45 years in the CLARITY study". Ectrims: P1204. Archived from the original on 2021-07-17. Retrieved 2021-10-27.
- ↑ Giovannoni, Gavin (2019). "Efficacy of Cladribine Tablets in high disease activity subgroups of patients with relapsing multiple sclerosis: A post hoc analysis of the CLARITY study". Mult Scler. 25 (6): 819–27. doi:10.1177/1352458518771875. PMC 6460686. PMID 29716436.
- ↑ Rammohan, K (2012). "Cladribine tablets for relapsing-remitting multiple sclerosis: Efficacy across patient subgroups from the phase III CLARITY study". Mult Scler Relat Disord. 1 (1): 49–54. doi:10.1016/j.msard.2011.08.006. PMID 25876451. Archived from the original on 2021-11-02. Retrieved 2021-10-27.
- ↑ De Stefano N et al. Mult Scler 2018;24:222–6. doi : 10.1177/1352458517690269 Archived 2021-07-16 at the Wayback Machine
External links
| External sites: | |
|---|---|
| Identifiers: |
|
- Pages using duplicate arguments in template calls
- Webarchive template wayback links
- CS1 maint: numeric names: authors list
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without UNII source
- Drugs missing an ATC code
- Drug has EMA link
- Drugboxes which contain changes to watched fields
- Articles with specifically marked weasel-worded phrases from October 2020
- Articles with invalid date parameter in template
- Orphan drugs
- Purine antagonists
- Purines
- Organochlorides
- Johnson & Johnson brands
- Merck brands
- RTT
- World Health Organization essential medicines