Panobinostat
 | |
| Names | |
|---|---|
| Trade names | Farydak |
| Other names | LBH-589 |
| |
| Clinical data | |
| Drug class | Histone deacetylase inhibitor (HDAC inhibitor)[1] |
| Main uses | Multiple myeloma[1] |
| Side effects | Diarrhea, tiredness, nausea, swelling, fever, weight loss, high magnesium, low calcium, low potassium, low white blood cells, low platelets[2] |
| Routes of use | By mouth (capsules) |
| Typical dose | 20 mg OD[1] |
| External links | |
| AHFS/Drugs.com | Monograph |
| Legal | |
| License data |
|
| Legal status |
|
| Pharmacokinetics | |
| Bioavailability | 21%[3] |
| Protein binding | 90%[3] |
| Metabolism | CYP3A (40%), CYP2D6, CYP2C19[3] |
| Elimination half-life | 37 hours[3] |
| Excretion | Fecal (44–77%), renal (29–51%)[3] |
| Chemical and physical data | |
| Formula | C21H23N3O2 |
| Molar mass | 349.434 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Panobinostat, sold under the brand name Farydak, is a medication used to treat multiple myeloma.[1] It is used when at least two other treatments have failed.[1] It is taken by mouth.[1] It is used together with bortezomib and dexamethasone.[1]
Common side effects include diarrhea, tiredness, nausea, swelling, fever, weight loss, high magnesium, low calcium, low potassium, low white blood cells, and low platelets.[2] Other side effects may include bleeding, QT prolongation, and infections.[2] Use during pregnancy may harm the baby.[2] It is a histone deacetylase inhibitor (HDAC inhibitor).[1]
Panobinostat was approved for medical use in the United States and Europe in 2015.[2][4] In the United Kingdom six doses costs the NHS about £4,700 as of 2021.[1] This amount in the United States costs about 15,000 USD.[5]
Medical uses
Panobinostat is used in combination with the anti-cancer drug bortezomib and the corticoid dexamethasone for the treatment of multiple myeloma in adults who had received at least two previous treatments, including bortezomib and an immunomodulatory agent.[6][7]: 660
Dosage
It is generally take as 20 mg once per day.[1] Out of 21 days it is taken on day 1, 3, 5, 8, 10, and 12.[1]
Contraindications
Panobinostat is contraindicated in nursing mothers. To judge from experiments in animals, there is a risk for the unborn child if used during pregnancy; still, the benefit of panobinostat may outweigh this risk.[8]
Side effects
Common side effects (in more than 10% of patients) include low blood cell counts (pancytopenia, thrombocytopenia, anaemia, leucopenia, neutropenia, lymphopenia), airway infections, as well as unspecific reactions such as fatigue, diarrhoea, nausea, headache, and sleeping problems.[8]
Pharmacology
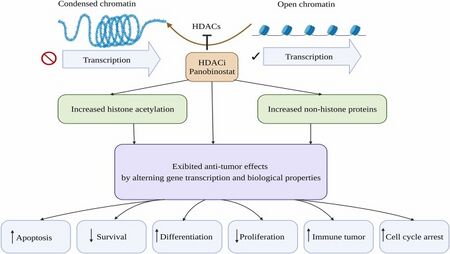
Mechanism of action
Panobinostat inhibits multiple histone deacetylase enzymes, a mechanism leading to apoptosis of malignant cells via multiple pathways.[10]
Pharmacokinetics
Panobinostat is absorbed quickly and almost completely from the gut, but has a significant first-pass effect, resulting in a total bioavailability of 21%. Highest blood plasma levels in patients with advanced cancer are reached after two hours. Plasma protein binding is about 90%. The substance is metabolised mainly through oxidation by the liver enzyme CYP3A4 and to a small extent by CYP2D6 and CYP2C19. It is also reduced, hydrolyzed and glucuronidized by unspecified enzymes. All metabolites seem to be inactive.[8]
Biological half-life is estimated to be 37 hours. 29–51% are excreted via the urine and 44–77% via the faeces.[8]
Research
Clinical trials
As of August 2012[update], it is being tested against Hodgkin's Lymphoma, cutaneous T cell lymphoma (CTCL)[11] and other types of malignant disease in Phase III clinical trials, against myelodysplastic syndromes, breast cancer and prostate cancer in Phase II trials, and against chronic myelomonocytic leukemia (CMML) in a Phase I trial.[12][13]
As of 2014[update] panobinostat is being used in a Phase I/II clinical trial that aims at curing AIDS in patients on highly active antiretroviral therapy (HAART). In this technique, panobinostat is used to drive the HIV DNA out of the patient's DNA, in the expectation that the patient's immune system in combination with HAART will destroy it.[14][15][16]
As of 2016[update] panobinostat is being studied in a phase II trial for relapsed and refractory diffuse large B-cell lymphoma (DLBCL).[17]
Preclinical studies
Panobinostat has been found to synergistically act with sirolimus to kill pancreatic cancer cells in the laboratory in a Mayo Clinic study. In the study, investigators found that this combination destroyed up to 65 percent of cultured pancreatic tumor cells. The finding is significant because the three cell lines studied were all resistant to the effects of chemotherapy – as are many pancreatic tumors.[18]
Panobinostat has also been found to significantly increase in vitro the survival of motor neuron (SMN) protein levels in cells of patients suffering from spinal muscular atrophy.[19]
Panobinostat was able to selectively target triple negative breast cancer (TNBC) cells by inducing hyperacetylation and cell cycle arrest at the G2-M DNA damage checkpoint; partially reversing the morphological changes characteristic of breast cancer cells.[20]
Panobinostat, along with other HDAC inhibitors, is also being studied for potential to induce virus HIV-1 expression in latently infected cells and disrupt latency. These resting cells are not recognized by the immune system as harboring the virus and do not respond to antiretroviral drugs.[21]
A 2015 study suggested Panobinostat was effective in preventing diffuse intrinsic pontine glioma cell growth in vitro and in vivo, identifying it as a potential drug candidate.[22]
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 979. ISBN 978-0857114105.
- ↑ 2.0 2.1 2.2 2.3 2.4 "Panobinostat Monograph for Professionals". Drugs.com. Archived from the original on 9 July 2021. Retrieved 26 October 2021.
- ↑ 3.0 3.1 3.2 3.3 3.4 "Panobinostat Package Insert" (PDF). Archived (PDF) from the original on 2021-04-10. Retrieved 2021-07-07.
- ↑ "Farydak product details". European Medicines Agency. Archived from the original on 2018-06-20. Retrieved 2022-03-14.
- ↑ "Farydak Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 11 May 2021. Retrieved 26 October 2021.
- ↑ "FDA.gov announcement about accelerated approval of panobinostat (Farydak)". Archived from the original on 2018-01-26. Retrieved 2021-07-07.
- ↑ Rajkumar, S. Vincent (2018). "Multiple Myeloma". In Hensley, Martee L.; Milowsky, Matthew I.; Rajkumar, S. Vincent; Schuetze, Scott M. (eds.). ASCO-SEP : Medical Oncology Self-Evaluation Program (7th ed.). Alexandria, VA: American Society of Clinical Oncology. ISBN 978-0-9983747-4-1. OCLC 1080368315. Archived from the original on 2021-10-17. Retrieved 2021-07-07.
- ↑ 8.0 8.1 8.2 8.3 Haberfeld, H, ed. (2016). Austria-Codex (in Deutsch). Vienna: Österreichischer Apothekerverlag.
- ↑ El Omari, Nasreddine; Bakrim, Saad; Khalid, Asaad; Abdalla, Ashraf N.; Almalki, Waleed Hassan; Lee, Learn-Han; Ardianto, Chrismawan; Ming, Long Chiau; Bouyahya, Abdelhakim (August 2023). "Molecular mechanisms underlying the clinical efficacy of panobinostat involve Stochasticity of epigenetic signaling, sensitization to anticancer drugs, and induction of cellular cell death related to cellular stresses". Biomedicine & Pharmacotherapy = Biomedecine & Pharmacotherapie. 164: 114886. doi:10.1016/j.biopha.2023.114886. ISSN 1950-6007.
- ↑ Revill P, Mealy N, Serradell N, Bolos J, Rosa E (2007). "Panobinostat". Drugs of the Future. 32 (4): 315. doi:10.1358/dof.2007.032.04.1094476.
- ↑ Clinical trial number NCT00425555 for "Study of Oral LBH589 in Adult Patients With Refractory Cutaneous T-Cell Lymphoma" at ClinicalTrials.gov
- ↑ "Studies found for LBH-589". ClinicalTrials.gov. Archived from the original on 2017-03-26. Retrieved 2021-07-07.
- ↑ Prince HM, Prince M (2009). "Panobinostat (LBH589): a novel pan-deacetylase inhibitor with activity in T cell lymphoma". Hematology Meeting Reports. Parkville, Australia: Peter MacCallum Cancer Centre and University of Melbourne. 3 (1): 33–38. Archived from the original on 2017-05-15. Retrieved 2021-07-07.
- ↑ Simons J (27 April 2013). "Scientists on brink of HIV cure". The Telegraph. Archived from the original on 27 April 2013.
- ↑ Clinical trial number NCT01680094 for "Safety and Effect of The HDAC Inhibitor Panobinostat on HIV-1 Expression in Patients on Suppressive HAART (CLEAR)" at ClinicalTrials.gov
- ↑ Rasmussen TA, Tolstrup M, Brinkmann CR, Olesen R, Erikstrup C, Solomon A, et al. (October 2014). "Panobinostat, a histone deacetylase inhibitor, for latent-virus reactivation in HIV-infected patients on suppressive antiretroviral therapy: a phase 1/2, single group, clinical trial". The Lancet. HIV. 1 (1): e13-21. doi:10.1016/S2352-3018(14)70014-1. PMID 26423811.
- ↑ Hoffman J (May 2016). "Panobinostat May Be Active in Select Patients With Refractory DLBCL". CancerTherapyAdvisor.com. Archived from the original on 2016-08-08. Retrieved 2021-07-07.
- ↑ "Mayo Clinic Researchers Formulate Treatment Combination Lethal To Pancreatic Cancer Cells". The Mayo Clinic. Archived from the original on 20 February 2012.
- ↑ Garbes L, Riessland M, Hölker I, Heller R, Hauke J, Tränkle C, et al. (October 2009). "LBH589 induces up to 10-fold SMN protein levels by several independent mechanisms and is effective even in cells from SMA patients non-responsive to valproate". Human Molecular Genetics. 18 (19): 3645–58. doi:10.1093/hmg/ddp313. PMID 19584083.
- ↑ Tate CR, Rhodes LV, Segar HC, Driver JL, Pounder FN, Burow ME, Collins-Burow BM (May 2012). "Targeting triple-negative breast cancer cells with the histone deacetylase inhibitor panobinostat". Breast Cancer Research. 14 (3): R79. doi:10.1186/bcr3192. PMC 3446342. PMID 22613095.
- ↑ Rasmussen TA, Schmeltz Søgaard O, Brinkmann C, Wightman F, Lewin SR, Melchjorsen J, et al. (May 2013). "Comparison of HDAC inhibitors in clinical development: effect on HIV production in latently infected cells and T-cell activation". Human Vaccines & Immunotherapeutics. 9 (5): 993–1001. doi:10.4161/hv.23800. PMC 3899169. PMID 23370291.
- ↑ Grasso CS, Tang Y, Truffaux N, Berlow NE, Liu L, Debily MA, et al. (June 2015). "Functionally defined therapeutic targets in diffuse intrinsic pontine glioma". Nature Medicine. 21 (6): 555–9. doi:10.1038/nm.3855. PMC 4862411. PMID 25939062.
External links
| External sites: | |
|---|---|
| Identifiers: |
|
- Pages using duplicate arguments in template calls
- CS1 Deutsch-language sources (de)
- Drugs with non-standard legal status
- Articles with changed InChI identifier
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without UNII source
- Drugs missing an ATC code
- Drug has EMA link
- Drugboxes which contain changes to verified fields
- Drugboxes which contain changes to watched fields
- Articles containing potentially dated statements from August 2012
- Articles with invalid date parameter in template
- All articles containing potentially dated statements
- Articles containing potentially dated statements from 2014
- Articles containing potentially dated statements from 2016
- Articles with changed DrugBank identifier
- Articles with changed ChemSpider identifier
- Articles with changed KEGG identifier
- Indoles
- Hydroxamic acids
- Histone deacetylase inhibitors
- Novartis brands
- Cancer treatments
- Orphan drugs
- RTT