Brodalumab
| Monoclonal antibody | |
|---|---|
| Type | Whole antibody |
| Source | Human |
| Target | Interleukin 17 receptor A |
| Names | |
| Trade names | Siliq, Kyntheum |
| Other names | KHK4827, AMG 827 |
| Clinical data | |
| Main uses | Plaque psoriasis[1] |
| Side effects | Joint pain, headache, tiredness, diarrhea, throat pain, nausea, low white blood cells, tinea infections[2] |
| Routes of use | SubQ[2] |
| Typical dose | 210 mg[2] |
| External links | |
| AHFS/Drugs.com | Monograph |
| Legal | |
| License data |
|
| Legal status |
|
| Chemical and physical data | |
| Formula | C6372H9840N1712O1988S52 |
| Molar mass | 143905.93 g·mol−1 |
Brodalumab, sold under the brand name Siliq and Kyntheum, is a medication used to treat plaque psoriasis.[1] Specifically it is used for moderate to severe disease in people who have not improved with other treatments.[2] It is given by injection under the skin.[2]
Common side effects include joint pain, headache, tiredness, diarrhea, throat pain, nausea, low white blood cells, and tinea infections.[2] Other side effects may include infection and Crohn's disease.[2] Safety in pregnancy is unclear.[2] It is a monoclonal antibody which blocks interleukin 17.[1]
Brodalumab was approved for medical use in the United States and Europe in 2017.[2][1] In the United Kingdom 4 weeks of medication costs the NHS about £1,300 as of 2021.[3] In the United States this amount costs about 2,800 USD.[4]
Medical uses
Dosage
It is given at a dose of 210 mg at time 0, 1 week, and 2 week.[2] It is than given every 2 weeks.[2]
Mechanism of action
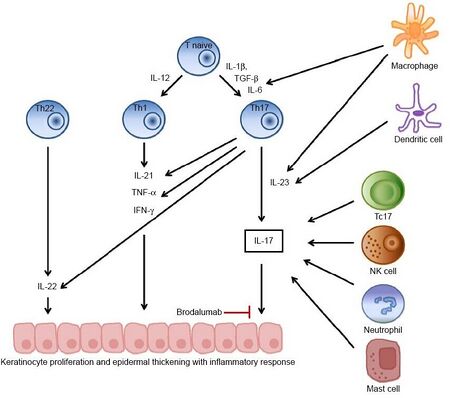
Brodalumab binds to the interleukin-17 receptor and so prevents interleukin 17 (IL-17) from activating the receptor. This mechanism is similar to that of another anti-psoriasis antibody, ixekizumab, which however binds to IL-17 itself.
History
Brodalumab was developed by Amgen, Inc. as AMG 827.
In 2013 it was in two phase III clinical trials for the treatment of moderate to severe psoriasis.[6][7]
In November 2014, Amgen and AstraZeneca reported encouraging results for the compound. The companies stated that the compound met the primary endpoint showing superior skin clearance in a Phase III trial when compared to ustekinumab and a placebo.[8]
However, in May 2015, Amgen announced that it was ending its participation in co-development of the compound because of reports of patients having "events of suicidal ideation and behavior".[9] AstraZeneca will be solely responsible for any future development and marketing of brodalumab in all territories except for certain Asian territories such as Japan, where Kyowa Hakko Kirin has rights to brodalumab and continued as KHK4827.
In September 2015, AstraZeneca announced a partnership with Valeant Pharmaceuticals in which Valeant took over exclusive rights to develop and commercialize brodalumab.[10] In July 2016, the rights to commercialize brodalumab in Europe were sold to LEO Pharma.[11]
In January 2016, a biologics license application (BLA) was submitted to the US FDA.[12] Approval followed in February 2017.[13]
References
- ↑ 1.0 1.1 1.2 1.3 "Kyntheum". Archived from the original on 13 September 2021. Retrieved 11 January 2022.
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 "DailyMed - SILIQ- brodalumab injection". dailymed.nlm.nih.gov. Archived from the original on 18 March 2021. Retrieved 11 January 2022.
- ↑ BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 1300. ISBN 978-0857114105.
- ↑ "Brodalumab Prices, Coupons & Savings Tips - GoodRx". GoodRx. Retrieved 11 January 2022.
- ↑ Roman, Michael; Chiu, Melvin W. (7 July 2017). "Spotlight on brodalumab in the treatment of moderate-to-severe plaque psoriasis: design, development, and potential place in therapy". Drug Design, Development and Therapy. 11: 2065–2075. doi:10.2147/DDDT.S113683.
- ↑ Clinical trial number NCT01708590 for "Study of Efficacy, Safety, and Withdrawal and Retreatment With Brodalumab in Moderate to Severe Plaque Psoriasis Subjects (AMAGINE-1)" at ClinicalTrials.gov
- ↑ Clinical trial number NCT01708629 for "Study of Efficacy and Safety of Brodalumab Compared With Placebo and Ustekinumab in Moderate to Severe Plaque Psoriasis Subjects (AMAGINE-3)" at ClinicalTrials.gov
- ↑ Philippidis A (26 November 2014). "Brodalumab's No Turkey in Phase III: Amgen and AstraZeneca". Genetic Engineering & Biotechnology News. Archived from the original on 2014-12-03. Retrieved 2014-11-27.
- ↑ "Amgen to terminate participation in co-development and commercialization of brodaluma". Amgen. Archived from the original on 2015-05-26. Retrieved 2021-10-13.
- ↑ "AstraZeneca and Valeant Pharmaceuticals to partner on brodalumab". www.astrazeneca.com. Archived from the original on 2021-10-04. Retrieved 2021-10-13.
- ↑ "AstraZeneca enters licensing agreements with LEO Pharma in skin diseases". www.astrazeneca.com. Archived from the original on 2021-10-03. Retrieved 2021-10-13.
- ↑ House DW (25 January 2016). "FDA accepts AstraZeneca's brodalumab BLA for plaque psoriasis, PDUFA date November 16". Seeking Alpha. Archived from the original on 8 February 2017. Retrieved 13 October 2021.
- ↑ Office of the Commissioner (15 February 2017). "Press Announcements - FDA approves new psoriasis drug". www.fda.gov. Archived from the original on 2 June 2020. Retrieved 18 February 2017.
External links
| Identifiers: |
|
|---|
- Pages using duplicate arguments in template calls
- Articles with hatnote templates targeting a nonexistent page
- Missing redirects
- Drugs with non-standard legal status
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Drugs missing an ATC code
- Drugboxes which contain changes to verified fields
- Drugboxes which contain changes to watched fields
- Drugs that are a monoclonal antibody
- Chemicals that do not have a ChemSpider ID assigned
- Articles with changed KEGG identifier
- Amgen
- Experimental drugs
- RTT
- All stub articles
- Antineoplastic and immunomodulating drug stubs
- Monoclonal antibody stubs