Ixekizumab
| Monoclonal antibody | |
|---|---|
| Type | Whole antibody |
| Source | Humanized |
| Target | Interleukin 17A (IL-17A) |
| Names | |
| Pronunciation | ix-ee-KIZ-ue-mab[1] |
| Trade names | Taltz |
| Clinical data | |
| Main uses | Plaque psoriasis, psoriatic arthritis, ankylosing spondylitis[2][3] |
| Side effects | Respiratory infections, injection site pain[2] |
| Pregnancy category | |
| Routes of use | Subcutaneous injection |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a616025 |
| Legal | |
| License data |
|
| Legal status | |
| Pharmacokinetics | |
| Bioavailability | 54–90% |
| Metabolism | Presumably proteolysis |
| Elimination half-life | 13 days |
| Chemical and physical data | |
| Formula | C6492H10012N1728O2028S46 |
| Molar mass | 146192.34 g·mol−1 |
Ixekizumab, sold under the brand name Taltz, is a medication used to treat plaque psoriasis, psoriatic arthritis, and ankylosing spondylitis.[2][3] For plaque psoriasis it is used for moderate to severe disease.[2] It is given as an injection under the skin.[2]
Common side effects include respiratory infections and injection site pain.[2] Other side effects may include infections and allergic reactions.[3] Safety in pregnancy is unclear.[6] It is a monoclonal antibody that attaches to and blocks interleukin 17A, reducing inflammation.[2]
Ixekizumab was approved for medical use in the Europe and the United States in 2016.[3][2] In the United Kingdom it costs the NHS about £1,130 per 80 mg dose as of 2021.[7] In the United States this amount costs about 6,250 USD.[8]
Medical uses
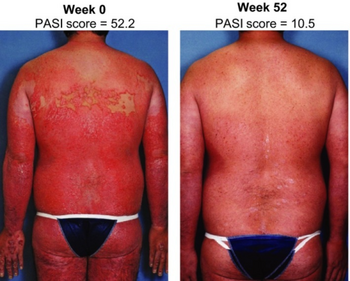
Ixekizumab is used to treat adults with moderate-to-severe plaque psoriasis who are candidates for systemic therapy or phototherapy, active psoriatic arthritis, active ankylosing spondylitis, and active non-radiographic axial spondyloarthritis with objective signs of inflammation.[9] It is also used for moderate-to-severe plaque psoriasis.[2][10]
In studies, the drug reduced the Psoriasis Area and Severity Index by at least 75% (PASI75) in 82–89% of patients during the first three months of treatment (depending on the dosing scheme), and 40% of patients experienced a complete absence of psoriasis symptoms (PASI100). In the placebo group, PASI75 was reached in 4% of patients, and PASI100 in none; in the group of patients receiving etanercept, an older anti-psoriasis drug, PASI75 was reached in 48%. Until the 60th study week, 11–44% of ixekizumab treated patients relapsed (again, depending on the dosing scheme), as compared to 84% under placebo.[10][11]
Dosage
It is given as an initial dose of 160 mg and than given at a dose of 80 every two to four weeks.[3]
Contraindications
The medication is contraindicated for patients with certain infections such as active tuberculosis.[10]
Side effects
In studies, ixekizumab increased the rate of infections (27% of ixekizumab treated patients, compared to 23% under placebo), including severe ones (0.6% versus 0.4% under placebo). Other common side effects included injection site pain (13–17% versus 3%), oropharyngeal pain (1%) and nausea (1–2%).[10]
Overdose
Up to fourfold doses have been given in studies without causing serious side effects.[10]
Interactions
No interaction studies have been done. Ixekizumab and interleukin 17 are not known to interact with cytochrome P450 (CYP) liver enzymes. Since inflammation suppresses CYP activity, it is theorized that ixekizumab could neutralize this effect and lower blood plasma concentrations of drugs that are metabolized by CYP enzymes, such as warfarin.[10]
Pharmacology
Mechanism of action
Ixekizumab binds to interleukin 17 (IL-17A), a pro-inflammatory cytokine, and blocks its action. Among other things, IL-17A stimulates proliferation and activation of keratinocytes in the skin.[10] This mechanism is similar to that of another anti-psoriasis antibody, brodalumab, which binds to the interleukin-17 receptor.[12]
The antibody has affinity to the homodimer IL-17A and the heterodimer IL-17A/F, but not to other members of the interleukin 17 family.[10]
Pharmacokinetics
After subcutaneous injection, ixekizumab has a bioavailability of 54–90%. Highest blood plasma concentrations are reached after four to seven days after a single dose. With the usual dosing scheme (loading plus a dose every two weeks), steady state concentrations are reached in the eighth week on average.[10]
Like other antibodies, ixekizumab is probably degraded by proteolysis. Its elimination half-life is 13 days.[10]
Chemistry
Ixekizumab is a complete monoclonal antibody of the subclass IgG4, consisting of two light chains and two heavy chains linked by disulfide bridges. Both heavy chains are glycosylated at the asparagine in position 296. In the hinge region, a serine is replaced by a proline to reduce formation of half-antibodies and heterodimers in the manufacturing process. The terminal lysine found in wild-type IgG4 is removed. The antibody is produced in Chinese hamster ovary cells.[13][14]
History
Clinical trials included a Phase II trial of patients with moderate to severe psoriasis,[12] and a Phase III open-label trial.[16][full citation needed]
Ixekizumab was approved by the US Food and Drug Administration (FDA) in March 2016, for the treatment of adults with moderate-to-severe plaque psoriasis[17] and by the European Medicines Agency (EMA) in April 2016.[2]
The safety and efficacy of ixekizumab were established in three randomized, placebo-controlled clinical trials with a total of 3,866 participants with plaque psoriasis who were candidates for systemic or phototherapy therapy.[17]
The FDA approved ixekizumab based on the evidence from three clinical trials of 1958 participants with moderate to severe psoriasis.[18]
The trials were conducted in the USA, Canada, Europe, Russia, Mexico, Chile, Argentina, Japan and Australia.[18]
In December 2017, the FDA approved it for active psoriatic arthritis.[19]
References
- ↑ "12 Difficult-to-Pronounce Drug Names". Pharmacy Times. 7 February 2018. Archived from the original on 21 March 2018. Retrieved 22 March 2018.
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 "Taltz EPAR". European Medicines Agency (EMA). 2 May 2016. Archived from the original on 27 March 2020. Retrieved 27 March 2020.
- ↑ 3.0 3.1 3.2 3.3 3.4 "Ixekizumab Monograph for Professionals". Drugs.com. Archived from the original on 4 March 2021. Retrieved 26 November 2021.
- ↑ 4.0 4.1 "Ixekizumab (Taltz) Use During Pregnancy". Drugs.com. 27 November 2019. Archived from the original on 27 March 2020. Retrieved 27 March 2020.
- ↑ "Taltz 80 mg solution for injection in pre-filled syringe - Summary of Product Characteristics (SmPC)". (emc). 21 August 2019. Archived from the original on 27 March 2020. Retrieved 27 March 2020.
- ↑ "Ixekizumab (Taltz) Use During Pregnancy". Drugs.com. Archived from the original on 27 March 2020. Retrieved 1 December 2021.
- ↑ BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 1302. ISBN 978-0857114105.
- ↑ "Taltz Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 16 January 2021. Retrieved 1 December 2021.
- ↑ "Taltz- ixekizumab injection, solution". DailyMed. 23 August 2019. Archived from the original on 2 July 2019. Retrieved 27 March 2020.
- ↑ 10.0 10.1 10.2 10.3 10.4 10.5 10.6 10.7 10.8 10.9 Haberfeld H, ed. (2015). Austria-Codex (in German). Vienna: Österreichischer Apothekerverlag.
{{cite book}}: CS1 maint: unrecognized language (link) - ↑ Klement A (4 June 2016). "Taltz". Österreichische Apothekerzeitung (in German) (14/2016): 12.
{{cite journal}}: CS1 maint: unrecognized language (link) - ↑ 12.0 12.1 "Neue Antikörper in der Pipeline". Pharmazeutische Zeitung (in German) (12). 2012. Archived from the original on 3 April 2016. Retrieved 24 September 2020.
{{cite journal}}: CS1 maint: unrecognized language (link) - ↑ "Statement On A Nonproprietary Name Adopted By The USAN Council: Ixekizumab" (PDF). American Medical Association. Archived (PDF) from the original on 31 October 2021. Retrieved 24 September 2020.
- ↑ "Assessment report: Taltz" (PDF). European Medicines Agency. 25 February 2016. p. 7. Archived (PDF) from the original on 27 March 2020. Retrieved 24 September 2020.
- ↑ Blauvelt, Andrew; Lebwohl, Mark G.; Mabuchi, Tomotaka; Leung, Ann; Garrelts, Alyssa; Crane, Heidi; ElMaraghy, Hany; Patel, Himanshu; Ridenour, Terri; See, Kyoungah; Gallo, Gaia; Paul, Carle (August 2021). "Long-term efficacy and safety of ixekizumab: A 5-year analysis of the UNCOVER-3 randomized controlled trial". Journal of the American Academy of Dermatology. 85 (2): 360–368. doi:10.1016/j.jaad.2020.11.022. ISSN 1097-6787.
- ↑ Clinical trial number NCT01624233 for "A Study in Japanese Participants With Moderate-to-Severe Psoriasis" at ClinicalTrials.gov
- ↑ 17.0 17.1 "FDA approves new psoriasis drug Taltz". U.S. Food and Drug Administration (FDA) (Press release). 22 March 2016. Archived from the original on 23 April 2019. Retrieved 27 March 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ 18.0 18.1 "Drug Trials Snapshots: Taltz". U.S. Food and Drug Administration (FDA). 22 March 2016. Archived from the original on 26 September 2020. Retrieved 24 September 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "FDA approves Taltz for psoriatic arthritis in adults". Healio. 4 December 2017. Archived from the original on 31 October 2021. Retrieved 23 September 2020.
External links
| External sites: | |
|---|---|
| Identifiers: |
|
- Pages using duplicate arguments in template calls
- CS1 maint: unrecognized language
- Wikipedia articles incorporating the PD-notice template
- Use dmy dates from March 2020
- Articles with invalid date parameter in template
- Drugs with non-standard legal status
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Drugs missing an ATC code
- Drug has EMA link
- Drugboxes which contain changes to verified fields
- Drugs that are a monoclonal antibody
- All articles with incomplete citations
- Articles with incomplete citations from September 2020
- Articles with changed CASNo identifier
- Chemicals that do not have a ChemSpider ID assigned
- Eli Lilly and Company brands
- Immunosuppressants
- Monoclonal antibodies
- RTT