Crohn's disease
| Crohn's disease | |
|---|---|
| Other names: Crohn disease, Crohn syndrome, granulomatous enteritis, regional enteritis, Leśniowski-Crohn disease | |
 | |
| The three most common sites of intestinal involvement in Crohn's disease | |
| Specialty | Gastroenterology |
| Symptoms | Abdominal pain, diarrhea (may be bloody), fever, weight loss[1] |
| Complications | Anemia, skin rashes, arthritis, bowel cancer[1] |
| Usual onset | 20 to 30[2] |
| Duration | Long term[1] |
| Risk factors | Tobacco smoking[3] |
| Diagnostic method | Biopsy, medical imaging[1] |
| Differential diagnosis | Irritable bowel syndrome, celiac disease, Behçet's disease, nonsteroidal anti-inflammatory drug enteropathy, intestinal tuberculosis[1][4] |
| Medication | Corticosteroids, methotrexate[1] |
| Prognosis | Slightly reduced life expectancy[1] |
| Frequency | 3.2 per 1,000 (developed world)[5] |
Crohn's disease is a type of inflammatory bowel disease (IBD) that may affect any segment of the gastrointestinal tract from the mouth to the anus.[2] Symptoms often include abdominal pain, diarrhea (which may be bloody if inflammation is severe), fever, and weight loss.[1][2] Other complications outside the gastrointestinal tract may include anemia, skin rashes, arthritis, inflammation of the eye, and tiredness.[1] The skin rashes may be due to infections as well as pyoderma gangrenosum or erythema nodosum.[1] Bowel obstruction may occur as a complication of chronic inflammation, and those with the disease are at greater risk of colon cancer and small bowel cancer.[1]
While the causes of Crohn's disease are unknown, it is believed to be caused by a combination of environmental, immune, and bacterial factors in genetically susceptible individuals.[6][7][8][2] It results in a chronic inflammatory disorder, in which the body's immune system attacks the gastrointestinal tract, possibly targeting microbial antigens.[7][9] While Crohn's is an immune-related disease, it does not appear to be an autoimmune disease (in that the immune system is not being triggered by the body itself).[10] The exact underlying immune problem is not clear; however, it may be an immunodeficiency state.[9][11][12] About half of the overall risk is related to genetics, with more than 70 genes having been found to be involved.[1][13] Tobacco smokers are twice as likely to develop Crohn's disease as nonsmokers.[3] It also often begins after gastroenteritis.[1] Diagnosis is based on a number of findings including biopsy and appearance of the bowel wall, medical imaging, and description of the disease.[1] Other conditions that can present similarly include irritable bowel syndrome and Behçet's disease.[1]
There are not any medications or surgical procedures that can cure Crohn's disease.[1][2] Treatment options are intended to help with symptoms, maintain remission, and prevent relapse.[1] In those newly diagnosed, a corticosteroid may be used for a brief period of time to rapidly improve symptoms, alongside another medication such as either methotrexate or a thiopurine used to prevent recurrence.[1] Stopping smoking is recommended in people with Crohn's disease.[1] One in five people with the disease is admitted to hospital each year, and half of those with the disease will require surgery for the disease at some point over a ten-year period.[1] While surgery should be used as little as possible, it is necessary to address some abscesses, certain bowel obstructions, and cancers.[1] Checking for bowel cancer via colonoscopy is recommended every few years, starting eight years after the disease has begun.[1]
Crohn's disease affects about 3.2 per 1,000 people in Europe and North America.[5] It is less common in Asia and Africa.[14][15] It has historically been more common in the developed world.[16] Rates have, however, been increasing, particularly in the developing world, since the 1970s.[15][16] Inflammatory bowel disease resulted in 47,400 deaths in 2015[17] and those with Crohn's disease have a slightly reduced life expectancy.[1] It tends to start in the teens and twenties, though it can occur at any age.[1][2][18] Males and females are equally affected.[2] The disease was named after gastroenterologist Burrill Bernard Crohn, who in 1932, together with two colleagues at Mount Sinai Hospital in New York, described a series of patients with inflammation of the terminal ileum of the small intestine, the area most commonly affected by the illness.[19]
Signs and symptoms
| Crohn's disease | Ulcerative colitis | |
|---|---|---|
| Defecation | Often porridge-like,[20] sometimes steatorrhea |
Often mucus-like and with blood[20] |
| Tenesmus | Less common[20] | More common[20] |
| Fever | Common[20] | Indicates severe disease[20] |
| Fistulae | Common[21] | Seldom |
| Weight loss | Often | More seldom |
Gastrointestinal

Many people with Crohn's disease have symptoms for years before the diagnosis.[22] The usual onset is in the teens and twenties, but can occur at any age.[18][1] Because of the 'patchy' nature of the gastrointestinal disease and the depth of tissue involvement, initial symptoms can be more subtle than those of ulcerative colitis. People with Crohn's disease experience chronic recurring periods of flare-ups and remission.[23]
Abdominal pain is a common initial symptom of Crohn's disease,[2] especially in the lower right abdomen.[24] It is often accompanied by diarrhea, which may or may not be bloody. Inflammation in different areas of the intestinal tract can affect the quality of the feces. Ileitis typically results in large-volume, watery feces, while colitis may result in a smaller volume of feces of higher frequency. Fecal consistency may range from solid to watery. In severe cases, an individual may have more than 20 bowel movements per day, and may need to awaken at night to defecate.[1][25][26][27] Visible bleeding in the feces is less common in Crohn's disease than in ulcerative colitis, but is not unusual.[1] Bloody bowel movements are usually intermittent, and may be bright or dark red in color. In severe Crohn's colitis, bleeding may be copious.[25] Flatulence and bloating may add to the intestinal discomfort.[25][28][29]
Symptoms caused by intestinal stenosis are also common in Crohn's disease. Abdominal pain is often most severe in areas of the bowel with stenoses. Persistent vomiting and nausea may indicate stenosis from small bowel obstruction or disease involving the stomach, pylorus, or duodenum.[25] Although the association is greater in the context of ulcerative colitis, Crohn's disease may also be associated with primary sclerosing cholangitis, a type of inflammation of the bile ducts.[30]
Perianal discomfort may also be prominent in Crohn's disease. Itchiness or pain around the anus may be suggestive of inflammation, fistulization or abscess around the anal area[1] or anal fissure. Perianal skin tags are also common in Crohn's disease, and may appear with or without the presence of colorectal polyps.[31] Fecal incontinence may accompany perianal Crohn's disease.
The mouth may be affected by recurrent sores (aphthous ulcers). Rarely, the esophagus and stomach may be involved in Crohn's disease. These can cause symptoms including difficulty swallowing (dysphagia), upper abdominal pain, and vomiting.[32]
Systemic
Like many other chronic, inflammatory diseases, Crohn's disease can cause a variety of systemic symptoms.[1] Among children, growth failure is common. Many children are first diagnosed with Crohn's disease based on inability to maintain growth.[33] As it may manifest at the time of the growth spurt in puberty, up to 30% of children with Crohn's disease may have retardation of growth.[34] Fever may also be present, though fevers greater than 38.5 °C (101.3 °F) are uncommon unless there is a complication such as an abscess.[1] Among older individuals, Crohn's disease may manifest as weight loss, usually related to decreased food intake, since individuals with intestinal symptoms from Crohn's disease often feel better when they do not eat and might lose their appetite.[33] People with extensive small intestine disease may also have malabsorption of carbohydrates or lipids, which can further exacerbate weight loss.[35]
Extraintestinal

Crohn's disease can affect many organ systems beyond the gastrointestinal tract.[36] Inflammation of the interior portion of the eye, known as uveitis, can cause blurred vision and eye pain, especially when exposed to light (photophobia).[37] Inflammation may also involve the white part of the eye (sclera) or the overlying connective tissue (episclera), which causes conditions called scleritis and episcleritis, respectively.[37] Uveitis can lead to loss of vision if untreated.[36]
Crohn's disease that affects the ileum may result in an increased risk of gallstones. This is due to a decrease in bile acid resorption in the ileum, and the bile gets excreted in the stool. As a result, the cholesterol/bile ratio increases in the gallbladder, resulting in an increased risk for gallstones.[37]
Crohn's disease is associated with a type of rheumatologic disease known as seronegative spondyloarthropathy.[37] This group of diseases is characterized by inflammation of one or more joints (arthritis) or muscle insertions (enthesitis).[37] The arthritis in Crohn's disease can be divided into two types. The first type affects larger weight-bearing joints such as the knee (most common), hips, shoulders, wrists, or elbows.[37] The second type symmetrically involves five or more of the small joints of the hands and feet.[37] The arthritis may also involve the spine, leading to ankylosing spondylitis if the entire spine is involved, or simply sacroiliitis if only the sacroiliac joint is involved.[37] The symptoms of arthritis include painful, warm, swollen, stiff joints, and loss of joint mobility or function.[38]
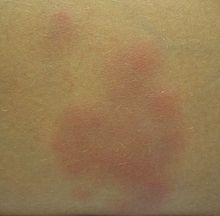
Crohn's disease may also involve the skin, blood, and endocrine system. Erythema nodosum is the most common type of skin problem, occurring in around 8% of people with Crohn's disease, producing raised, tender red nodules usually appearing on the shins.[37][39][40] Erythema nodosum is due to inflammation of the underlying subcutaneous tissue, and is characterized by septal panniculitis.[39] Pyoderma gangrenosum is a less common skin problem, occurring in under 2%,[40] and is typically a painful ulcerating nodule.[39][36]
Crohn's disease also increases the risk of blood clots;[37] painful swelling of the lower legs can be a sign of deep venous thrombosis, while difficulty breathing may be a result of pulmonary embolism. Autoimmune hemolytic anemia, a condition in which the immune system attacks the red blood cells, is also more common in Crohn's disease and may cause fatigue, a pale appearance, and other symptoms common in anemia. Clubbing, a deformity of the ends of the fingers, may also be a result of Crohn's disease. Finally, Crohn's disease increases the risk of osteoporosis, or thinning of the bones.[37] Individuals with osteoporosis are at increased risk of bone fractures.[41]
People with Crohn's disease may develop anemia due to vitamin B12, folate, iron deficiency, or due to anemia of chronic disease.[42][43] The most common is iron deficiency anemia[42] from chronic blood loss, reduced dietary intake, and persistent inflammation leading to increased hepcidin levels, restricting iron absorption in the duodenum.[43] As Crohn's disease most commonly affects the terminal ileum where the vitamin B12/intrinsic factor complex is absorbed, B12 deficiency may be seen.[43] This is particularly common after surgery to remove the ileum.[42] Involvement of the duodenum and jejunum can impair the absorption of many other nutrients including folate. People with Crohn's often also have issues with small bowel bacterial overgrowth syndrome, which can produce micronutrient deficiencies.[44]
Crohn's disease can also cause neurological complications (reportedly in up to 15%).[45] The most common of these are seizures, stroke, myopathy, peripheral neuropathy, headache, and depression.[45]
Mouth
Recurrent aphthous ulcers are common; however, it is not clear whether this is due to Crohn's disease or simply that they are common in the general population.[46] Other findings may include diffuse or nodular swelling of the mouth, a cobblestone appearance inside the mouth, granulomatous ulcers, or pyostomatitis vegetans.[47][48] Medications that are commonly prescribed to treat CD, such as anti-inflammatory and sulfa-containing drugs, may cause lichenoid drug reactions in the mouth.[47] Fungal infection such as candidiasis is also common, and people have a higher risk of cavities.[47] Signs of anemia such as pallor and angular cheilitis or glossitis are also common.[47]
Complications
| Crohn's disease |
Ulcerative colitis | ||
|---|---|---|---|
| Nutrient deficiency | Higher risk | ||
| Colon cancer risk | Slight | Considerable | |
| Percent of people with extraintestinal complications[49][50][51] | |||
| Iritis/uveitis | Females | 2.2% | 3.2% |
| Males | 1.3% | 0.9% | |
| Primary sclerosing cholangitis |
Females | 0.3% | 1% |
| Males | 0.4% | 3% | |
| Ankylosing spondylitis |
Females | 0.7% | 0.8% |
| Males | 2.7% | 1.5% | |
| Pyoderma gangrenosum |
Females | 1.2% | 0.8% |
| Males | 1.3% | 0.7% | |
| Erythema nodosum | Females | 1.9% | 2% |
| Males | 0.6% | 0.7% | |
Crohn's disease can lead to several mechanical complications within the intestines, including obstruction,[52] fistulae,[53] and abscesses.[54] Obstruction typically occurs from strictures or adhesions that narrow the lumen, blocking the passage of the intestinal contents. A fistula can develop between two loops of bowel, between the bowel and bladder, between the bowel and vagina, and between the bowel and skin. Abscesses are walled-off concentrations of infection, which can occur in the abdomen or in the perianal area. Crohn's is responsible for 10% of vesicoenteric fistulae, and is the most common cause of ileovesical fistulae.[55]
Crohn's disease also increases the risk of cancer in the area of inflammation. For example, individuals with Crohn's disease involving the small bowel are at higher risk for small intestinal cancer.[56] Similarly, people with Crohn's colitis have a relative risk of 5.6 for developing colon cancer.[57] Screening for colon cancer with colonoscopy is recommended for anyone who has had Crohn's colitis for at least eight years.[58]

Some studies suggest there is a role for chemoprotection in the prevention of colorectal cancer in Crohn's involving the colon; two agents have been suggested, folate and mesalamine preparations.[59] Also, immunomodulators and biologic agents used to treat this disease may promote developing extra-intestinal cancers.[60]
Individuals with Crohn's disease are at risk of malnutrition for many reasons, including decreased food intake and malabsorption. The risk increases following resection of the small bowel. Such individuals may require oral supplements to increase their caloric intake, or in severe cases, total parenteral nutrition (TPN). Most people with moderate or severe Crohn's disease are referred to a dietitian for assistance in nutrition.[61]
The major significant complications of Crohn's disease include bowel obstruction, abscesses, free perforation, and hemorrhage, which in rare cases may be fatal.[62][63]
Crohn's disease can be problematic during pregnancy, and some medications can cause adverse outcomes for the fetus or mother. Consultation with an obstetrician and gastroenterologist about Crohn's disease and all medications facilitates preventative measures. In some cases, remission occurs during pregnancy. Certain medications can also lower sperm count or otherwise adversely affect a man's fertility.[64]
Cause
| Crohn's disease | Ulcerative colitis | |
|---|---|---|
| Smoking | Higher risk for smokers | Lower risk for smokers[65] |
| Age | Usual onset between 15 and 30 years[66] |
Peak incidence between 15 and 25 years |
While the exact cause is unknown, Crohn's disease seems to be due to a combination of environmental factors and genetic predisposition.[67] Crohn's is the first genetically complex disease in which the relationship between genetic risk factors and the immune system is understood in considerable detail.[68] Each individual risk mutation makes a small contribution to the overall risk of Crohn's (approximately 1:200). The genetic data, and direct assessment of immunity, indicates a malfunction in the innate immune system.[69] In this view, the chronic inflammation of Crohn's is caused when the adaptive immune system tries to compensate for a deficient innate immune system.[70]
Genetics

Crohn's has a genetic component.[72] Because of this, siblings of known people with Crohn's are 30 times more likely to develop Crohn's than the general population.[citation needed]
The first mutation found to be associated with Crohn's was a frameshift in the NOD2 gene (also known as the CARD15 gene),[73] followed by the discovery of point mutations.[74] Over 30 genes have been associated with Crohn's; a biological function is known for most of them. For example, one association is with mutations in the XBP1 gene, which is involved in the unfolded protein response pathway of the endoplasmic reticulum.[75][76] The gene variants of NOD2/CARD15 seem to be related with small-bowel involvement.[77] Other well documented genes which increase the risk of developing Crohn disease are ATG16L1,[78] IL23R,[79] IRGM,[80] and SLC11A1.[81] There is considerable overlap between susceptibility loci for IBD and mycobacterial infections.[82] Genome-wide association studies have shown that Crohn's disease is genetically linked to coeliac disease.[83]
Crohn's has been linked to the gene LRRK2 with one variant potentially increasing the risk of developing the disease by 70%, while another lowers it by 25%. The gene is responsible for making a protein, which collects and eliminates waste product in cells, and is also associated with Parkinson's disease.[84]
Immune system
There was a prevailing view that Crohn's disease is a primary T cell autoimmune disorder; however, a newer theory hypothesizes that Crohn's results from an impaired innate immunity.[85] The later hypothesis describes impaired cytokine secretion by macrophages, which contributes to impaired innate immunity and leads to a sustained microbial-induced inflammatory response in the colon, where the bacterial load is high.[7][69] Another theory is that the inflammation of Crohn's was caused by an overactive Th1 and Th17 cytokine response.[86][87]
In 2007, the ATG16L1 gene was implicated in Crohn's disease, which may induce autophagy and hinder the body's ability to attack invasive bacteria.[78] Another study theorized that the human immune system traditionally evolved with the presence of parasites inside the body, and that the lack thereof due to modern hygiene standards has weakened the immune system. Test subjects were reintroduced to harmless parasites, with positive response.[88]
Microbes
It is hypothesised that maintenance of commensal microorganism growth in the GI tract is dysregulated, either as a result or cause of immune dysregulation.[89][90]
A number of studies have suggested a causal role for Mycobacterium avium subspecies paratuberculosis (MAP), which causes a similar disease, Johne's disease, in cattle.[91][92]
NOD2 is a gene involved in Crohn's genetic susceptibility. It is associated with macrophages' diminished ability to phagocytize MAP. This same gene may reduce innate and adaptive immunity in gastrointestinal tissue and impair the ability to resist infection by the MAP bacterium.[93] Macrophages that ingest the MAP bacterium are associated with high production of TNF-α.[94][95]
Other studies have linked specific strains of enteroadherent E. coli to the disease.[96] Adherent-invasive Escherichia coli (AIEC), are more common in people with CD,[97][98][96] have the ability to make strong biofilms compared to non-AIEC strains correlating with high adhesion and invasion indices[99][100] of neutrophils and the ability to block autophagy at the autolysosomal step, which allows for intracellular survival of the bacteria and induction of inflammation.[101] Inflammation drives the proliferation of AIEC and dysbiosis in the ileum, irrespective of genotype.[102] AIEC strains replicate extensively inside macrophages inducing the secretion of very large amounts of TNF-α.[103]
Mouse studies have suggested some symptoms of Crohn's disease, ulcerative colitis, and irritable bowel syndrome have the same underlying cause. Biopsy samples taken from the colons of all three patient groups were found to produce elevated levels of a serine protease.[104] Experimental introduction of the serine protease into mice has been found to produce widespread pain associated with irritable bowel syndrome, as well as colitis, which is associated with all three diseases.[105] Regional and temporal variations in those illnesses follow those associated with infection with the protozoan Blastocystis.[106]
The "cold-chain" hypothesis is that psychrotrophic bacteria such as Yersinia and Listeria species contribute to the disease. A statistical correlation was found between the advent of the use of refrigeration in the United States and various parts of Europe and the rise of the disease.[107][108][109]
There is an apparent connection between Crohn's disease, Mycobacterium, other pathogenic bacteria, and genetic markers.[110][111] In many individuals, genetic factors predispose individuals to Mycobacterium avium subsp. paratuberculosis infection. This bacterium then produces mannins, which protect both itself and various bacteria from phagocytosis, thereby causing a variety of secondary infections.[112]
Still, this relationship between specific types of bacteria and Crohn's disease remains unclear.[113][114]
There is a tentative association between Candida colonization and Crohn's disease.[115]
Environmental factors
The increased incidence of Crohn's in the industrialized world indicates an environmental component. Crohn's is associated with an increased intake of animal protein, milk protein, and an increased ratio of omega-6 to omega-3 polyunsaturated fatty acids.[116] Those who consume vegetable proteins appear to have a lower incidence of Crohn's disease. Consumption of fish protein has no association.[116] Smoking increases the risk of the return of active disease (flares).[3] The introduction of hormonal contraception in the United States in the 1960s is associated with a dramatic increase in incidence, and one hypothesis is that these drugs work on the digestive system in ways similar to smoking.[117] Isotretinoin is associated with Crohn's.[118][119][120]
Although stress is sometimes claimed to exacerbate Crohn's disease, there is no concrete evidence to support such claim.[2] Dietary microparticles, such as those found in toothpaste, have been studied as they produce effects on immunity, but they were not consumed in greater amounts in patients with Crohn's.[121][122] The use of doxycycline has also been associated with increased risk of developing inflammatory bowel diseases.[123][124][125] In one large retrospective study, patients who were prescribed doxycycline for their acne had a 2.25-fold greater risk of developing Crohn's disease.[124]
Pathophysiology
| Crohn's disease | Ulcerative colitis | |
|---|---|---|
| Cytokine response | Associated with Th17[126] | Vaguely associated with Th2 |
During a colonoscopy, biopsies of the colon are often taken to confirm the diagnosis. Certain characteristic features of the pathology seen point toward Crohn's disease; it shows a transmural pattern of inflammation, meaning the inflammation may span the entire depth of the intestinal wall.[1] Ulceration is an outcome seen in highly active disease. There is usually an abrupt transition between unaffected tissue and the ulcer—a characteristic sign known as skip lesions. Under a microscope, biopsies of the affected colon may show mucosal inflammation, characterized by focal infiltration of neutrophils, a type of inflammatory cell, into the epithelium. This typically occurs in the area overlying lymphoid aggregates. These neutrophils, along with mononuclear cells, may infiltrate the crypts, leading to inflammation (crypititis) or abscess (crypt abscess).
Granulomas, aggregates of macrophage derivatives known as giant cells, are found in 50% of cases and are most specific for Crohn's disease. The granulomas of Crohn's disease do not show "caseation", a cheese-like appearance on microscopic examination characteristic of granulomas associated with infections, such as tuberculosis. Biopsies may also show chronic mucosal damage, as evidenced by blunting of the intestinal villi, atypical branching of the crypts, and a change in the tissue type (metaplasia). One example of such metaplasia, Paneth cell metaplasia, involves development of Paneth cells (typically found in the small intestine and a key regulator of intestinal microbiota) in other parts of the gastrointestinal system.[127][128]
Diagnosis
The diagnosis of Crohn's disease can sometimes be challenging,[22] and a number of tests are often required to assist the physician in making the diagnosis.[25] Even with a full battery of tests, it may not be possible to diagnose Crohn's with complete certainty; a colonoscopy is approximately 70% effective in diagnosing the disease, with further tests being less effective. Disease in the small bowel is particularly difficult to diagnose, as a traditional colonoscopy allows access to only the colon and lower portions of the small intestines; introduction of the capsule endoscopy[129] aids in endoscopic diagnosis. Giant (multinucleate) cells, a common finding in the lesions of Crohn's disease, are less common in the lesions of lichen nitidus.[130]
-
Endoscopic image of Crohn's colitis showing deep ulceration
-
CT scan showing Crohn's disease in the fundus of the stomach
-
Endoscopic biopsy showing granulomatous inflammation of the colon in a case of Crohn's disease.
-
Section of colectomy showing transmural inflammation
-
Resected ileum from a person with Crohn's disease
Classification
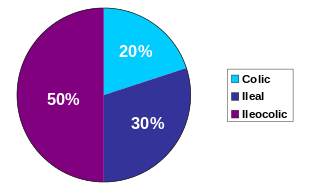
Crohn's disease is one type of inflammatory bowel disease (IBD). It typically manifests in the gastrointestinal tract and can be categorized by the specific tract region affected. A disease of both the ileum (the last part of the small intestine that connects to the large intestine), and the large intestine, Ileocolic Crohn's accounts for fifty percent of cases. Crohn's ileitis, manifest in the ileum only, accounts for thirty percent of cases, while Crohn's colitis, of the large intestine, accounts for the remaining twenty percent of cases and may be particularly difficult to distinguish from ulcerative colitis.
Gastroduodenal Crohn's disease causes inflammation in the stomach and first part of the small intestine called the duodenum. Jejunoileitis causes spotty patches of inflammation in the top half of the small intestine, called the jejunum.[131] The disease can attack any part of the digestive tract, from mouth to anus. However, individuals affected by the disease rarely fall outside these three classifications, with presentations in other areas.[1]
Crohn's disease may also be categorized by the behavior of disease as it progresses. These categorizations formalized in the Vienna classification of the disease.[132] There are three categories of disease presentation in Crohn's disease: stricturing, penetrating, and inflammatory. Stricturing disease causes narrowing of the bowel that may lead to bowel obstruction or changes in the caliber of the feces. Penetrating disease creates abnormal passageways (fistulae) between the bowel and other structures, such as the skin. Inflammatory disease (or nonstricturing, nonpenetrating disease) causes inflammation without causing strictures or fistulae.[132][133]
Endoscopy
A colonoscopy is the best test for making the diagnosis of Crohn's disease, as it allows direct visualization of the colon and the terminal ileum, identifying the pattern of disease involvement. On occasion, the colonoscopy can travel past the terminal ileum, but it varies from person to person. During the procedure, the gastroenterologist can also perform a biopsy, taking small samples of tissue for laboratory analysis, which may help confirm a diagnosis. As 30% of Crohn's disease involves only the ileum,[1] cannulation of the terminal ileum is required in making the diagnosis. Finding a patchy distribution of disease, with involvement of the colon or ileum, but not the rectum, is suggestive of Crohn's disease, as are other endoscopic stigmata.[134] The utility of capsule endoscopy for this, however, is still uncertain.[135] A "cobblestone"-like appearance is seen in approximately 40% of cases of Crohn's disease upon colonoscopy, representing areas of ulceration separated by narrow areas of healthy tissue.
Radiologic tests
A small bowel follow-through may suggest the diagnosis of Crohn's disease and is useful when the disease involves only the small intestine. Because colonoscopy and gastroscopy allow direct visualization of only the terminal ileum and beginning of the duodenum, they cannot be used to evaluate the remainder of the small intestine. As a result, a barium follow-through X-ray, wherein barium sulfate suspension is ingested and fluoroscopic images of the bowel are taken over time, is useful for looking for inflammation and narrowing of the small bowel.[134][136] Barium enemas, in which barium is inserted into the rectum and fluoroscopy is used to image the bowel, are rarely used in the work-up of Crohn's disease due to the advent of colonoscopy. They remain useful for identifying anatomical abnormalities when strictures of the colon are too small for a colonoscope to pass through, or in the detection of colonic fistulae (in this case contrast should be performed with iodate substances).[137]
CT and MRI scans are useful for evaluating the small bowel with enteroclysis protocols.[138] They are also useful for looking for intra-abdominal complications of Crohn's disease, such as abscesses, small bowel obstructions, or fistulae.[139] Magnetic resonance imaging (MRI) is another option for imaging the small bowel as well as looking for complications, though it is more expensive and less readily available.[140] MRI techniques such as diffusion-weighted imaging and high-resolution imaging are more sensitive in detecting ulceration and inflammation compared to CT.[141][142]
Blood tests
A complete blood count may reveal anemia, which commonly is caused by blood loss leading to iron deficiency or by vitamin B12 deficiency, usually caused by ileal disease impairing vitamin B12 absorption. Rarely autoimmune hemolysis may occur.[143] Ferritin levels help assess if iron deficiency is contributing to the anemia. Erythrocyte sedimentation rate (ESR) and C-reactive protein help assess the degree of inflammation, which is important as ferritin can also be raised in inflammation.[144] Serum iron, total iron binding capacity and transferrin saturation may be more easily interpreted in inflammation. Anemia of chronic disease results in a normocytic anemia.
Other causes of anemia include medication used in treatment of inflammatory bowel disease, like azathioprine, which can lead to cytopenia, and sulfasalazine, which can also result in folate deficiency. Testing for Saccharomyces cerevisiae antibodies (ASCA) and antineutrophil cytoplasmic antibodies (ANCA) has been evaluated to identify inflammatory diseases of the intestine[145] and to differentiate Crohn's disease from ulcerative colitis.[146] Furthermore, increasing amounts and levels of serological antibodies such as ASCA, antilaminaribioside [Glc(β1,3)Glb(β); ALCA], antichitobioside [GlcNAc(β1,4)GlcNAc(β); ACCA], antimannobioside [Man(α1,3)Man(α)AMCA], antiLaminarin [(Glc(β1,3))3n(Glc(β1,6))n; anti-L] and antichitin [GlcNAc(β1,4)n; anti-C] associate with disease behavior and surgery, and may aid in the prognosis of Crohn's disease.[147][148][149][150]
Low serum levels of vitamin D are associated with Crohn's disease.[151] Further studies are required to determine the significance of this association.[151]
Comparison with ulcerative colitis
The most common disease that mimics the symptoms of Crohn's disease is ulcerative colitis, as both are inflammatory bowel diseases that can affect the colon with similar symptoms. It is important to differentiate these diseases, since the course of the diseases and treatments may be different. In some cases, however, it may not be possible to tell the difference, in which case the disease is classified as indeterminate colitis.[1][25][26]
| Crohn's disease | Ulcerative colitis | |
|---|---|---|
| Terminal ileum involvement | Commonly | Seldom |
| Colon involvement | Usually | Always |
| Rectum involvement | Seldom | Usually (95%)[152] |
| Involvement around the anus |
Common[21] | Seldom |
| Bile duct involvement | No increase in rate of primary sclerosing cholangitis | Higher rate[153] |
| Distribution of disease | Patchy areas of inflammation (skip lesions) | Continuous area of inflammation[152] |
| Endoscopy | Deep geographic and serpiginous (snake-like) ulcers | Continuous ulcer |
| Depth of inflammation | May be transmural, deep into tissues[21][154] | Shallow, mucosal |
| Stenosis | Common | Seldom |
| Granulomas on biopsy | May have non-necrotizing non-peri-intestinal crypt granulomas[21][155][156] | Non-peri-intestinal crypt granulomas not seen[157] |
Differential diagnosis
Other conditions with similar symptoms as Crohn's disease includes intestinal tuberculosis, Behçet's disease, ulcerative colitis, nonsteroidal anti-inflammatory drug enteropathy, irritable bowel syndrome and celiac disease.[4] Irritable bowel syndrome is excluded when there are inflammatory changes.[4] Celiac disease cannot be excluded if specific antibodies (anti-transglutaminase antibodies) are negative,[158][159] nor in absence of intestinal villi atrophy.[160][161]
Management
| Crohn's disease | Ulcerative colitis | |
|---|---|---|
| Mesalazine | Less useful[162] | More useful[162] |
| Antibiotics | Effective in long-term[163] | Generally not useful[164] |
| Surgery | Often returns following removal of affected part |
Usually cured by removal of colon |
There is no cure for Crohn's disease and remission may not be possible or prolonged if achieved. In cases where remission is possible, relapse can be prevented and symptoms controlled with medication, lifestyle and dietary changes, changes to eating habits (eating smaller amounts more often), reduction of stress, moderate activity and exercise. Surgery is generally contraindicated and has not been shown to prevent remission. Adequately controlled, Crohn's disease may not significantly restrict daily living.[165] Treatment for Crohn's disease is only when symptoms are active and involve first treating the acute problem, then maintaining remission.
Lifestyle changes
Certain lifestyle changes can reduce symptoms, including dietary adjustments, elemental diet, proper hydration, and smoking cessation. Diets that include higher levels of fiber and fruit are associated with reduced risk, while diets rich in total fats, polyunsaturated fatty acids, meat, and omega-6 fatty acids may increase the risk of Crohn's.[166] Maintaining a balanced diet with proper portion control can help manage symptoms of the disease. Eating small meals frequently instead of big meals may also help with a low appetite. A food diary may help with identifying foods that trigger symptoms. Some people should follow a low fiber diet to control acute symptoms especially if fibrous foods cause symptoms.[165] Some find relief in eliminating casein (protein found in cow's milk) and gluten (protein found in wheat, rye and barley) from their diets. They may have specific dietary intolerances (not allergies).[167] Fatigue can be helped with regular exercise, a healthy diet, and enough sleep. Smoking may worsen symptoms, and stopping is recommended.[165]
Medication
Acute treatment uses medications to treat any infection (normally antibiotics) and to reduce inflammation (normally aminosalicylate anti-inflammatory drugs and corticosteroids). When symptoms are in remission, treatment enters maintenance, with a goal of avoiding the recurrence of symptoms. Prolonged use of corticosteroids has significant side-effects; as a result, they are, in general, not used for long-term treatment. Alternatives include aminosalicylates alone, though only a minority are able to maintain the treatment, and many require immunosuppressive drugs.[21] It has been also suggested that antibiotics change the enteric flora, and their continuous use may pose the risk of overgrowth with pathogens such as Clostridium difficile.[168]
Medications used to treat the symptoms of Crohn's disease include 5-aminosalicylic acid (5-ASA) formulations, prednisone, immunomodulators such as azathioprine (given as the prodrug for 6-mercaptopurine), methotrexate, infliximab, adalimumab,[26] certolizumab,[169] vedolizumab, ustekinumab,[170] and natalizumab.[171][172] Hydrocortisone should be used in severe attacks of Crohn's disease.[173] Biological therapies are medications used to avoid long-term steroid use, decrease inflammation, and treat people who have fistulas with abscesses.[24] The monoclonal antibody ustekinumab appears to be a safe treatment option, and may help people with moderate to severe active Crohn's disease.[174] The long term safety and effectiveness of monoclonal antibody treatment is not known.[174] The monoclonal antibody briakinumab is not effective for people with active Crohn's disease and it is no longer being manufactured.[174]
The gradual loss of blood from the gastrointestinal tract, as well as chronic inflammation, often leads to anemia, and professional guidelines suggest routinely monitoring for this.[175][176][177] Adequate disease control usually improves anemia of chronic disease, but iron deficiency may require treatment with iron supplements. Guidelines vary as to how iron should be administered. Besides other, problems include a limitation in possible daily resorption and an increased growth of intestinal bacteria. Some[177] advise parenteral iron as first line as it works faster, has fewer gastrointestinal side effects, and is unaffected by inflammation reducing enteral absorption.
Other guidelines[176] advise oral iron as first line with parenteral iron reserved for those that fail to adequately respond as oral iron is considerably cheaper. All agree that severe anemia (hemoglobin under 10g/dL) should be treated with parenteral iron. Blood transfusion should be reserved for those who are cardiovascularly unstable, due to its relatively poor safety profile, lack of long term efficacy, and cost.[176]
Surgery
Crohn's cannot be cured by surgery, as the disease eventually recurs, though it is used in the case of partial or full blockage of the intestine.[178] Surgery may also be required for complications such as obstructions, fistulas, or abscesses, or if the disease does not respond to drugs. After the first surgery, Crohn's usually comes back at the site where the diseased intestine was removed and the healthy ends were rejoined, however it can come back in other locations. After a resection, scar tissue builds up, which can cause strictures, which form when the intestines become too small to allow excrement to pass through easily, which can lead to a blockage. After the first resection, another resection may be necessary within five years.[179] For patients with an obstruction due to a stricture, two options for treatment are strictureplasty and resection of that portion of bowel. There is no statistical significance between strictureplasty alone versus strictureplasty and resection in cases of duodenal involvement. In these cases, re-operation rates were 31% and 27%, respectively, indicating that strictureplasty is a safe and effective treatment for selected people with duodenal involvement.[180]
Postsurgical recurrence of Crohn's disease is relatively common. Crohn's lesions are nearly always found at the site of the resected bowel. The join (or anastomosis) after surgery may be inspected, usually during a colonoscopy, and disease activity graded. The "Rutgeert's score" is an endoscopic scoring system for post-operative disease recurrence in Crohn's disease. Mild postsurgical recurrences of Crohn's disease are graded i1 and i2, moderate to severe recurrences are graded i3 and i4.[181] Fewer lesions result in a lower grade. Based on the score, treatment plans can be designed to give the patient the best chance of managing recurrence of the disease.[182]
Short bowel syndrome (SBS, also short gut syndrome or simply short gut) is caused by the surgical removal of part of the small intestine. It usually develops in those patients who have had half or more of their small intestines removed.[183] Diarrhea is the main symptom, but others may include weight loss, cramping, bloating, and heartburn. Short bowel syndrome is treated with changes in diet, intravenous feeding, vitamin and mineral supplements, and treatment with medications. In some cases of SBS, intestinal transplant surgery may be considered; though the number of transplant centres offering this procedure is quite small and it comes with a high risk due to the chance of infection and rejection of the transplanted intestine.[184]
Bile acid diarrhea is another complication following surgery for Crohn's disease in which the terminal ileum has been removed. This leads to the development of excessive watery diarrhea. It is usually thought to be due to an inability of the ileum to reabsorb bile acids after resection of the terminal ileum and was the first type of bile acid malabsorption recognized.[185]
Mental health
Crohn's may result in anxiety or mood disorders, especially in young people who may have stunted growth or embarrassment from fecal incontinence.[186] Counselling as well as antidepressant or anxiolytic medication may help some people manage.[186]
As of 2017[update] there is a small amount of research looking at mindfulness-based therapies, hypnotherapy, and cognitive behavioural therapy.[187]
Alternative medicine
It is common for people with Crohn's disease to try complementary or alternative therapy.[188] These include diets, probiotics, fish oil and other herbal and nutritional supplements.
- Acupuncture is used to treat inflammatory bowel disease in China, and is being used more frequently in Western society.[189] At this time, evidence is insufficient to recommend the use of acupuncture.[188]
- A 2006 survey in Germany found that about half of people with IBD used some form of alternative medicine, with the most common being homeopathy, and a study in France found that about 30% used alternative medicine.[190] Homeopathic preparations are not proven with this or any other condition,[191][192][193][192][193] with large-scale studies finding them to be no more effective than a placebo.[194][195][196]
- There are contradicting studies regarding the effect of medical cannabis on inflammatory bowel disease,[197] and its effects on management are uncertain.[198]
Prognosis
Crohn's disease is a chronic condition for which there is no known cure. It is characterised by periods of improvement followed by episodes when symptoms flare up. With treatment, most people achieve a healthy weight, and the mortality rate for the disease is relatively low. It can vary from being benign to very severe, and people with CD could experience just one episode or have continuous symptoms. It usually reoccurs, although some people can remain disease-free for years or decades. Most people with Crohn's live a normal lifespan.[199] However, Crohn's disease is associated with a small increase in risk of small bowel and colorectal carcinoma (bowel cancer).[200]
Epidemiology
The percentage of people with Crohn's disease has been determined in Norway and the United States and is similar at 6 to 7.1:100,000. The Crohn's and Colitis Foundation of America cites this number as approx 149:100,000; NIH cites 28 to 199 per 100,000.[201][202] Crohn's disease is more common in northern countries, and with higher rates still in the northern areas of these countries.[203] The incidence of Crohn's disease is thought to be similar in Europe but lower in Asia and Africa.[201] It also has a higher incidence in Ashkenazi Jews[1][204] and smokers.[205]
Crohn's disease begins most commonly in people in their teens and 20s, and people in their 50s through to their 70s.[1][25][18] It is rarely diagnosed in early childhood. It usually affects female children more severely than males.[206] However, only slightly more women than men have Crohn's disease.[207] Parents, siblings or children of people with Crohn's disease are 3 to 20 times more likely to develop the disease.[208] Twin studies find that if one has the disease there is a 55% chance the other will too.[209]
The incidence of Crohn's disease is increasing in Europe[210] and in newly industrialised countries.[211] For example, in Brazil, there has been an annual increase of 11% in the incidence of Crohn's disease since 1990.[211]
History
Inflammatory bowel diseases were described by Giovanni Battista Morgagni (1682–1771) and by Scottish physician T Kennedy Dalzie in 1913.[212]
Ileitis terminalis was first described by Polish surgeon Antoni Leśniowski in 1904, although it was not conclusively distinguished from intestinal tuberculosis.[213] In Poland, it is still called Leśniowski-Crohn's disease (Polish: choroba Leśniowskiego-Crohna). Burrill Bernard Crohn, an American gastroenterologist at New York City's Mount Sinai Hospital, described fourteen cases in 1932, and submitted them to the American Medical Association under the rubric of "Terminal ileitis: A new clinical entity". Later that year, he, along with colleagues Leon Ginzburg and Gordon Oppenheimer, published the case series as "Regional ileitis: a pathologic and clinical entity". However, due to the precedence of Crohn's name in the alphabet, it later became known in the worldwide literature as Crohn's disease.[19]
Research
Some evidence supports the hypothesis that the bacterium Mycobacterium avium subspecies paratuberculosis (MAP) is a cause of Crohn's disease (see also Johne's disease). As a result, researchers are looking at the eradication of MAP as a therapeutic option.[214] Treating MAP using antibiotics has been examined and the results are unclear but tentatively beneficial.[215][216]
Crohn's is common in parts of the world where helminthic colonisation is rare and uncommon in those areas where most people carry worms. Infections with helminths may alter the autoimmune response that causes the disease. Trials of extracts from the worm Trichuris suis showed promising results when used in people with IBD.[217][218][219] However these trials (TRUST -I & TRUST -II) failed in Phase 2 clinical trials and were then discontinued after consistent failure in both North America and Europe.[220][221]
There is no good evidence that thalidomide or lenalidomide is useful to bring about or maintain remission.[222][223]
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 1.16 1.17 1.18 1.19 1.20 1.21 1.22 1.23 1.24 1.25 1.26 1.27 1.28 1.29 1.30 1.31 1.32 1.33 1.34 1.35 Baumgart DC, Sandborn WJ (August 2012). "Crohn's disease". Lancet. 380 (9853): 1590–605. doi:10.1016/S0140-6736(12)60026-9. PMID 22914295.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 2.8 "Crohn's Disease". National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Archived from the original on December 8, 2019. Retrieved December 8, 2019.
- ↑ 3.0 3.1 3.2 Cosnes J (June 2004). "Tobacco and IBD: relevance in the understanding of disease mechanisms and clinical practice". Best Practice & Research. Clinical Gastroenterology. 18 (3): 481–96. doi:10.1016/j.bpg.2003.12.003. PMID 15157822.
- ↑ 4.0 4.1 4.2 "Inflammatory Bowel Disease" (PDF). World Gastroenterology Organization. August 2015. Archived (PDF) from the original on March 14, 2016. Retrieved March 13, 2016.
- ↑ 5.0 5.1 Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema HW, Kaplan GG (January 2012). "Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review". Gastroenterology. 142 (1): 46–54.e42, quiz e30. doi:10.1053/j.gastro.2011.10.001. PMID 22001864. S2CID 206223870.[permanent dead link]
- ↑ Cho JH, Brant SR (May 2011). "Recent insights into the genetics of inflammatory bowel disease". Gastroenterology. 140 (6): 1704–12. doi:10.1053/j.gastro.2011.02.046. PMC 4947143. PMID 21530736.
- ↑ 7.0 7.1 7.2 Dessein R, Chamaillard M, Danese S (September 2008). "Innate immunity in Crohn's disease: the reverse side of the medal". Journal of Clinical Gastroenterology. 42 Suppl 3 Pt 1: S144–7. doi:10.1097/MCG.0b013e3181662c90. PMID 18806708.
- ↑ Stefanelli T, Malesci A, Repici A, Vetrano S, Danese S (May 2008). "New insights into inflammatory bowel disease pathophysiology: paving the way for novel therapeutic targets". Current Drug Targets. 9 (5): 413–8. doi:10.2174/138945008784221170. PMID 18473770.
- ↑ 9.0 9.1 Marks DJ, Rahman FZ, Sewell GW, Segal AW (February 2010). "Crohn's disease: an immune deficiency state". Clinical Reviews in Allergy & Immunology. 38 (1): 20–31. doi:10.1007/s12016-009-8133-2. PMC 4568313. PMID 19437144.
- ↑ Casanova JL, Abel L (August 2009). "Revisiting Crohn's disease as a primary immunodeficiency of macrophages". The Journal of Experimental Medicine. 206 (9): 1839–43. doi:10.1084/jem.20091683. PMC 2737171. PMID 19687225.
- ↑ Lalande JD, Behr MA (July 2010). "Mycobacteria in Crohn's disease: how innate immune deficiency may result in chronic inflammation". Expert Review of Clinical Immunology. 6 (4): 633–41. doi:10.1586/eci.10.29. PMID 20594136.
- ↑ Yamamoto-Furusho JK, Korzenik JR (November 2006). "Crohn's disease: innate immunodeficiency?". World Journal of Gastroenterology. 12 (42): 6751–5. doi:10.3748/wjg.v12.i42.6751. PMC 4087427. PMID 17106921. Archived from the original on June 6, 2013.
- ↑ Barrett JC, Hansoul S, Nicolae DL, Cho JH, Duerr RH, Rioux JD, et al. (August 2008). "Genome-wide association defines more than 30 distinct susceptibility loci for Crohn's disease". Nature Genetics. 40 (8): 955–62. doi:10.1038/ng.175. PMC 2574810. PMID 18587394.
- ↑ Prideaux L, Kamm MA, De Cruz PP, Chan FK, Ng SC (August 2012). "Inflammatory bowel disease in Asia: a systematic review". Journal of Gastroenterology and Hepatology. 27 (8): 1266–80. doi:10.1111/j.1440-1746.2012.07150.x. PMID 22497584.
- ↑ 15.0 15.1 Hovde Ø, Moum BA (April 2012). "Epidemiology and clinical course of Crohn's disease: results from observational studies". World Journal of Gastroenterology. 18 (15): 1723–31. doi:10.3748/wjg.v18.i15.1723. PMC 3332285. PMID 22553396.
- ↑ 16.0 16.1 Burisch J, Munkholm P (July 2013). "Inflammatory bowel disease epidemiology". Current Opinion in Gastroenterology. 29 (4): 357–62. doi:10.1097/MOG.0b013e32836229fb. PMID 23695429.
- ↑ GBD 2015 Mortality Causes of Death Collaborators (October 2016). "Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015". The Lancet. 388 (10053): 1459–1544. doi:10.1016/S0140-6736(16)31012-1. PMC 5388903. PMID 27733281.
- ↑ 18.0 18.1 18.2 "Crohn's Disease: Get Facts on Symptoms and Diet". eMedicineHealth. Archived from the original on October 20, 2007.
- ↑ 19.0 19.1 Crohn BB, Ginzburg L, Oppenheimer GD (May 2000). "Regional ileitis: a pathologic and clinical entity. 1932". The Mount Sinai Journal of Medicine, New York. 67 (3): 263–8. PMID 10828911.
- ↑ 20.0 20.1 20.2 20.3 20.4 20.5 internetmedicin.se > Inflammatorisk tarmsjukdom, kronisk, IBD By Robert Löfberg. Retrieved Oct 2010 Translate.
- ↑ 21.0 21.1 21.2 21.3 21.4 Hanauer SB, Sandborn W (March 1, 2001). "Management of Crohn's disease in adults" (PDF). American Journal of Gastroenterology. 96 (3): 635–43. doi:10.1111/j.1572-0241.2001.03671.x. PMID 11280528. Retrieved November 7, 2009.
- ↑ 22.0 22.1 Pimentel M, Chang M, Chow EJ, Tabibzadeh S, Kirit-Kiriak V, Targan SR, Lin HC (December 2000). "Identification of a prodromal period in Crohn's disease but not ulcerative colitis". The American Journal of Gastroenterology. 95 (12): 3458–62. PMID 11151877.
- ↑ National Research Council (2003). "Johne's Disease and Crohn's Disease". Diagnosis and Control of Johne's Disease. Washington, DC: National Academies Press. doi:10.17226/10625. ISBN 978-0-309-08611-0. PMID 25032299. Bookshelf ID: NBK207651. Archived from the original on September 6, 2017. Retrieved August 7, 2020.
- ↑ 24.0 24.1 "What I need to know about Crohn's Disease". www.niddk.nih.gov. Archived from the original on November 21, 2015. Retrieved December 11, 2015.
- ↑ 25.0 25.1 25.2 25.3 25.4 25.5 25.6 Crohn Disease at eMedicine
- ↑ 26.0 26.1 26.2 Podolsky DK (August 2002). "Inflammatory bowel disease". The New England Journal of Medicine (Submitted manuscript). 347 (6): 417–29. doi:10.1056/NEJMra020831. PMID 12167685. Archived from the original on April 28, 2021. Retrieved August 7, 2020.
- ↑ Mueller MH, Kreis ME, Gross ML, Becker HD, Zittel TT, Jehle EC (August 2002). "Anorectal functional disorders in the absence of anorectal inflammation in patients with Crohn's disease". The British Journal of Surgery. 89 (8): 1027–31. doi:10.1046/j.1365-2168.2002.02173.x. PMID 12153630.
- ↑ Götz, Vera Nina (December 1, 2013). "Crohn's disease—What the medical registrar and acute physician needs to know!". Journal of Acute Medicine. 3 (4): 132–137. doi:10.1016/j.jacme.2013.10.001. Archived from the original on July 27, 2020. Retrieved August 7, 2020 – via ScienceDirect.
- ↑ Nóbrega, Viviane Gomes; Silva, Isaac Neri de Novais; Brito, Beatriz Silva; Silva, Juliana; Silva, Maria Carolina Martins da; Santana, Genoile Oliveira; Nóbrega, Viviane Gomes; Silva, Isaac Neri de Novais; Brito, Beatriz Silva; Silva, Juliana; Silva, Maria Carolina Martins da; Santana, Genoile Oliveira (September 27, 2018). "THE ONSET OF CLINICAL MANIFESTATIONS IN INFLAMMATORY BOWEL DISEASE PATIENTS". Arquivos de Gastroenterologia. 55 (3): 290–295. doi:10.1590/s0004-2803.201800000-73. Archived from the original on July 27, 2020. Retrieved August 7, 2020 – via SciELO.
- ↑ Robbins and Cotran: Pathologic Basis of Disease. 7th ed. Philadelphia, Pennsylvania: Elsevier Saunders; July 30, 2004. ISBN 978-0-7216-0187-8. The Gastrointestinal Tract. p. 847.
- ↑ Taylor BA, Williams GT, Hughes LE, Rhodes J (August 1989). "The histology of anal skin tags in Crohn's disease: an aid to confirmation of the diagnosis". International Journal of Colorectal Disease. 4 (3): 197–9. doi:10.1007/BF01649703. PMID 2769004.
- ↑ Fix OK, Soto JA, Andrews CW, Farraye FA (December 2004). "Gastroduodenal Crohn's disease". Gastrointestinal Endoscopy. 60 (6): 985. doi:10.1016/S0016-5107(04)02200-X. PMID 15605018.
- ↑ 33.0 33.1 Beattie RM, Croft NM, Fell JM, Afzal NA, Heuschkel RB (May 2006). "Inflammatory bowel disease". Archives of Disease in Childhood. 91 (5): 426–32. doi:10.1136/adc.2005.080481. PMC 2082730. PMID 16632672.
- ↑ Büller HA (February 1997). "Problems in diagnosis of IBD in children". The Netherlands Journal of Medicine (Submitted manuscript). 50 (2): S8–11. doi:10.1016/S0300-2977(96)00064-2. PMID 9050326. Archived from the original on August 28, 2021. Retrieved August 7, 2020.
- ↑ O'Keefe SJ (1996). "Nutrition and gastrointestinal disease". Scandinavian Journal of Gastroenterology. Supplement. 220: 52–9. doi:10.3109/00365529609094750. PMID 8898436.
- ↑ 36.0 36.1 36.2 Harbord, M; Annese, V; Vavricka, SR; Allez, M; Barreiro-de Acosta, M; Boberg, KM; Burisch, J; De Vos, M; De Vries, AM; Dick, AD; Juillerat, P; Karlsen, TH; Koutroubakis, I; Lakatos, PL; Orchard, T; Papay, P; Raine, T; Reinshagen, M; Thaci, D; Tilg, H; Carbonnel, F; European Crohn’s and Colitis, Organisation. (March 2016). "The First European Evidence-based Consensus on Extra-intestinal Manifestations in Inflammatory Bowel Disease". Journal of Crohn's & colitis. 10 (3): 239–54. doi:10.1093/ecco-jcc/jjv213. PMID 26614685.
- ↑ 37.00 37.01 37.02 37.03 37.04 37.05 37.06 37.07 37.08 37.09 37.10 Trikudanathan G, Venkatesh PG, Navaneethan U (December 2012). "Diagnosis and therapeutic management of extra-intestinal manifestations of inflammatory bowel disease". Drugs. 72 (18): 2333–49. doi:10.2165/11638120-000000000-00000. PMID 23181971.
- ↑ "Arthritis". Healthline Networks, Inc. October 10, 2008. Archived from the original on July 21, 2010. Retrieved August 16, 2010.
- ↑ 39.0 39.1 39.2 Thrash B, Patel M, Shah KR, Boland CR, Menter A (February 2013). "Cutaneous manifestations of gastrointestinal disease: part II". Journal of the American Academy of Dermatology. 68 (2): 211.e1–33, quiz 244–6. doi:10.1016/j.jaad.2012.10.036. PMID 23317981.
- ↑ 40.0 40.1 Roth, N; Biedermann, L; Fournier, N; Butter, M; Vavricka, SR; Navarini, AA; Rogler, G; Scharl, M; Swiss IBD Cohort Study Group. (2019). "Occurrence of skin manifestations in patients of the Swiss Inflammatory Bowel Disease Cohort Study". PLOS One. 14 (1): e0210436. doi:10.1371/journal.pone.0210436. PMID 30682031.
- ↑ Bernstein M, Irwin S, Greenberg GR (September 2005). "Maintenance infliximab treatment is associated with improved bone mineral density in Crohn's disease". The American Journal of Gastroenterology. 100 (9): 2031–5. PMID 16128948.
- ↑ 42.0 42.1 42.2 Lomer MC (August 2011). "Dietary and nutritional considerations for inflammatory bowel disease". The Proceedings of the Nutrition Society. 70 (3): 329–35. doi:10.1017/S0029665111000097. PMID 21450124.
- ↑ 43.0 43.1 43.2 Gerasimidis K, McGrogan P, Edwards CA (August 2011). "The aetiology and impact of malnutrition in paediatric inflammatory bowel disease". Journal of Human Nutrition and Dietetics (Review). 24 (4): 313–26. doi:10.1111/j.1365-277X.2011.01171.x. PMID 21564345.
- ↑ MedlinePlus Encyclopedia: Small bowel bacterial overgrowth
- ↑ 45.0 45.1 Crohn's disease Archived August 5, 2007, at the Wayback Machine. professionals.epilepsy.com. Retrieved July 13, 2007.
- ↑ Brad W. Neville, Douglas D. Damm, Carl M. Allen, Angela C. Chi (2016). Oral and Maxillofacial Pathology 4th Edition. Canada: Elsevier. p. 798. ISBN 978-1-4557-7052-6.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ↑ 47.0 47.1 47.2 47.3 Martin S. Greenberg, Michael Glick, Jonathan A. Ship (2008). Burket's Oral Medicine 11th Edition. India: BC Decker Inc. p. 355. ISBN 978-1-55009-345-2.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ↑ Zadik Y, Drucker S, Pallmon S (August 2011). "Migratory stomatitis (ectopic geographic tongue) on the floor of the mouth". Journal of the American Academy of Dermatology. 65 (2): 459–460. doi:10.1016/j.jaad.2010.04.016. PMID 21763590.
- ↑ Greenstein AJ, Janowitz HD, Sachar DB (September 1976). "The extra-intestinal complications of Crohn's disease and ulcerative colitis: a study of 700 patients". Medicine (Baltimore). 55 (5): 401–12. doi:10.1097/00005792-197609000-00004. PMID 957999.
- ↑ Bernstein, Charles N; Blanchard, James F; Rawsthorne, Patricia; Yu, Nancy (April 2001). "The Prevalence of Extraintestinal Diseases in Inflammatory Bowel Disease: A Population-Based Study:". American Journal of Gastroenterology. 96 (4): 1116–1122. doi:10.1111/j.1572-0241.2001.03756.x. PMID 11316157.
- ↑ Harbord, Marcus; Annese, Vito; Vavricka, Stephan R.; Allez, Matthieu; Barreiro-de Acosta, Manuel; Boberg, Kirsten Muri; Burisch, Johan; De Vos, Martine; De Vries, Anne-Marie; Dick, Andrew D.; Juillerat, Pascal; Karlsen, Tom H.; Koutroubakis, Ioannis; Lakatos, Peter L.; Orchard, Tim; Papay, Pavol; Raine, Tim; Reinshagen, Max; Thaci, Diamant; Tilg, Herbert; Carbonnel, Franck (March 1, 2016). "The First European Evidence-based Consensus on Extra-intestinal Manifestations in Inflammatory Bowel Disease". Journal of Crohn's and Colitis. 10 (3): 239–254. doi:10.1093/ecco-jcc/jjv213. PMID 26614685.
- ↑ "Intestinal Obstruction". MERCK MANUAL Consumer Version. Archived from the original on July 10, 2016. Retrieved June 27, 2016.
- ↑ "Anorectal Fistula". MERCK MANUAL Consumer Version. Archived from the original on July 10, 2016. Retrieved June 27, 2016.
- ↑ "Anorectal Abscess". MERCK MANUAL Consumer Version. Archived from the original on June 14, 2016. Retrieved June 27, 2016.
- ↑ Enterovesical Fistula at eMedicine
- ↑ Strategies for detecting colon cancer in patients with inflammatory bowel disease. The Cochrane Database of Systematic Reviews. 2017;9:CD000279. doi:10.1002/14651858.CD000279.pub4. PMID 28922695.
- ↑ Ekbom A, Helmick C, Zack M, Adami HO (August 1990). "Increased risk of large-bowel cancer in Crohn's disease with colonic involvement". Lancet. 336 (8711): 357–9. doi:10.1016/0140-6736(90)91889-I. PMID 1975343. Archived from the original on August 5, 2020. Retrieved August 7, 2020.
- ↑ Consensus conference: Colorectal cancer screening and surveillance in inflammatory bowel disease. Inflammatory Bowel Diseases. 2005;11(3):314–321. doi:10.1097/01.mib.0000160811.76729.d5. PMID 15735438.
- ↑ Zisman TL, Rubin DT (May 2008). "Colorectal cancer and dysplasia in inflammatory bowel disease". World Journal of Gastroenterology. 14 (17): 2662–9. doi:10.3748/wjg.14.2662. PMC 2709054. PMID 18461651.
- ↑ Axelrad JE, Lichtiger S, Yajnik V (May 2016). "Inflammatory bowel disease and cancer: The role of inflammation, immunosuppression, and cancer treatment". World Journal of Gastroenterology (Review). 22 (20): 4794–801. doi:10.3748/wjg.v22.i20.4794. PMC 4873872. PMID 27239106.
- ↑ Evans JP, Steinhart AH, Cohen Z, McLeod RS (2003). "Home total parenteral nutrition: an alternative to early surgery for complicated inflammatory bowel disease". Journal of Gastrointestinal Surgery. 7 (4): 562–566. doi:10.1016/S1091-255X(02)00132-4. PMID 12763417.
- ↑ Man of Many Problems Comes to City for Help. Richmond Times-Dispatch (Richmond, Virginia, USA). September 1, 1985:B1.
- ↑ "Kay, Laura Lynn". Richmond Times-Dispatch. Richmond, Virginia, USA. April 3, 2014.
Doris L. Johnson, 82, of Westminster. Carroll County Times (Westminster, Maryland, USA). March 2, 2014.
In memoriam: Dan Hodges Jr.. The Roanoke Times (Roanoke, Virginia, USA). December 31, 2013.
"Cynthia Meredith Routt". Daily Press. Newport News, Virginia, USA. May 4, 2014. p. A11. - ↑ Kaplan C (October 21, 2005). "IBD and Pregnancy: What You Need to Know". Crohn's and Colitis Foundation of America. Archived from the original on February 17, 2012. Retrieved November 7, 2009.
- ↑ Rubin DT, Ananthakrishnan AN, Siegel CA, Sauer BG, Long MD (March 2019). "ACG Clinical Guideline: Ulcerative Colitis in Adults". Am. J. Gastroenterol. 114 (3): 384–413. doi:10.14309/ajg.0000000000000152. PMID 30840605.

- ↑ Nachimuthu, Senthil. "Crohn's disease". eMedicineHealth. Archived from the original on December 9, 2019. Retrieved December 8, 2019.
- ↑ Braat H, Peppelenbosch MP, Hommes DW (August 2006). "Immunology of Crohn's disease". Annals of the New York Academy of Sciences. 1072 (1): 135–54. Bibcode:2006NYASA1072..135B. doi:10.1196/annals.1326.039. PMID 17057196.
- ↑ Henckaerts L, Figueroa C, Vermeire S, Sans M (May 2008). "The role of genetics in inflammatory bowel disease". Current Drug Targets. 9 (5): 361–8. doi:10.2174/138945008784221161. PMID 18473763.
- ↑ 69.0 69.1 Marks DJ, Harbord MW, MacAllister R, Rahman FZ, Young J, Al-Lazikani B, Lees W, Novelli M, Bloom S, Segal AW (February 2006). "Defective acute inflammation in Crohn's disease: a clinical investigation". Lancet. 367 (9511): 668–78. doi:10.1016/S0140-6736(06)68265-2. PMID 16503465.
- ↑ Comalada M, Peppelenbosch MP (September 2006). "Impaired innate immunity in Crohn's disease". Trends in Molecular Medicine. 12 (9): 397–9. doi:10.1016/j.molmed.2006.07.005. PMID 16890491.
- ↑ Nakagome S, Mano S, Kozlowski L, Bujnicki JM, Shibata H, Fukumaki Y, et al. (June 2012). "Crohn's disease risk alleles on the NOD2 locus have been maintained by natural selection on standing variation". Molecular Biology and Evolution. 29 (6): 1569–85. doi:10.1093/molbev/mss006. PMC 3697811. PMID 22319155.
- ↑ "Crohn's disease has strong genetic link: study". Crohn's and Colitis Foundation of America. April 16, 2007. Archived from the original on May 2, 2007. Retrieved November 7, 2009.
- ↑ Ogura Y, Bonen DK, Inohara N, Nicolae DL, Chen FF, Ramos R, et al. (May 2001). "A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease" (PDF). Nature. 411 (6837): 603–6. Bibcode:2001Natur.411..603O. doi:10.1038/35079114. hdl:2027.42/62856. PMID 11385577. Archived from the original on June 2, 2020. Retrieved August 7, 2020.
- ↑ Cuthbert AP, Fisher SA, Mirza MM, King K, Hampe J, Croucher PJ, Mascheretti S, Sanderson J, Forbes A, Mansfield J, Schreiber S, Lewis CM, Mathew CG (April 2002). "The contribution of NOD2 gene mutations to the risk and site of disease in inflammatory bowel disease". Gastroenterology. 122 (4): 867–74. doi:10.1053/gast.2002.32415. PMID 11910337.
- ↑ Kaser A, Lee AH, Franke A, Glickman JN, Zeissig S, Tilg H, et al. (September 2008). "XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease". Cell. 134 (5): 743–56. doi:10.1016/j.cell.2008.07.021. PMC 2586148. PMID 18775308.
- ↑ Clevers H (February 2009). "Inflammatory bowel disease, stress, and the endoplasmic reticulum". The New England Journal of Medicine. 360 (7): 726–7. doi:10.1056/NEJMcibr0809591. PMID 19213688.
- ↑ Vermeire S (June 2004). "NOD2/CARD15: relevance in clinical practice". Best Practice & Research. Clinical Gastroenterology (Review). 18 (3): 569–75. doi:10.1016/j.bpg.2003.12.008. PMID 15157828.
- ↑ 78.0 78.1 Prescott NJ, Fisher SA, Franke A, Hampe J, Onnie CM, Soars D, Bagnall R, Mirza MM, Sanderson J, Forbes A, Mansfield JC, Lewis CM, Schreiber S, Mathew CG (May 2007). "A nonsynonymous SNP in ATG16L1 predisposes to ileal Crohn's disease and is independent of CARD15 and IBD5". Gastroenterology. 132 (5): 1665–71. doi:10.1053/j.gastro.2007.03.034. PMID 17484864.
- ↑ Diegelmann J, Czamara D, Le Bras E, Zimmermann E, Olszak T, Bedynek A, et al. (2013). "Intestinal DMBT1 expression is modulated by Crohn's disease-associated IL23R variants and by a DMBT1 variant which influences binding of the transcription factors CREB1 and ATF-2". PLOS ONE. 8 (11): e77773. Bibcode:2013PLoSO...877773D. doi:10.1371/journal.pone.0077773. PMC 3818382. PMID 24223725.
- ↑ Prescott NJ, Dominy KM, Kubo M, Lewis CM, Fisher SA, Redon R, et al. (May 2010). "Independent and population-specific association of risk variants at the IRGM locus with Crohn's disease". Human Molecular Genetics. 19 (9): 1828–39. doi:10.1093/hmg/ddq041. PMC 2850616. PMID 20106866.
- ↑ Chermesh I, Azriel A, Alter-Koltunoff M, Eliakim R, Karban A, Levi BZ (July 2007). "Crohn's disease and SLC11A1 promoter polymorphism". Digestive Diseases and Sciences. 52 (7): 1632–5. doi:10.1007/s10620-006-9682-3. PMID 17385031.
- ↑ Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KY, et al. (November 2012). "Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease". Nature. 491 (7422): 119–24. Bibcode:2012Natur.491..119.. doi:10.1038/nature11582. PMC 3491803. PMID 23128233.
- ↑ Walker MM, Murray JA (August 2011). "An update in the diagnosis of coeliac disease". Histopathology (Review). 59 (2): 166–79. doi:10.1111/j.1365-2559.2010.03680.x. PMID 21054494.
Recent genome-wide association studies have shown that chronic inflammatory and autoimmune diseases are linked genetically to coeliac disease; for example, type 1 diabetes mellitus, Grave's disease and Crohn's disease.
- ↑ "A single gene can raise or lower Crohn's disease risk". New Scientist. January 20, 2018.
- ↑ Marks DJ, Segal AW (January 2008). "Innate immunity in inflammatory bowel disease: a disease hypothesis". The Journal of Pathology. 214 (2): 260–6. doi:10.1002/path.2291. PMC 2635948. PMID 18161747.
- ↑ Cobrin GM, Abreu MT (August 2005). "Defects in mucosal immunity leading to Crohn's disease". Immunological Reviews. 206 (1): 277–95. doi:10.1111/j.0105-2896.2005.00293.x. PMID 16048555.
- ↑ Elson CO, Cong Y, Weaver CT, Schoeb TR, McClanahan TK, Fick RB, Kastelein RA (June 2007). "Monoclonal anti-interleukin 23 reverses active colitis in a T cell-mediated model in mice". Gastroenterology. 132 (7): 2359–70. doi:10.1053/j.gastro.2007.03.104. PMID 17570211.
- ↑ The Worm Turns. The New York Times. June 29, 2008 [archived January 7, 2017].
- ↑ Sartor RB (July 2006). "Mechanisms of disease: pathogenesis of Crohn's disease and ulcerative colitis". Nature Clinical Practice. Gastroenterology & Hepatology. 3 (7): 390–407. doi:10.1038/ncpgasthep0528. PMID 16819502.
- ↑ Dogan B, Scherl E, Bosworth B, Yantiss R, Altier C, McDonough PL, Jiang ZD, Dupont HL, Garneau P, Harel J, Rishniw M, Simpson KW (January 2013). "Multidrug resistance is common in Escherichia coli associated with ileal Crohn's disease". Inflammatory Bowel Diseases. 19 (1): 141–50. doi:10.1002/ibd.22971. PMID 22508665.
- ↑ Naser SA, Collins MT (December 2005). "Debate on the lack of evidence of Mycobacterium avium subsp. paratuberculosis in Crohn's disease". Inflammatory Bowel Diseases. 11 (12): 1123. doi:10.1097/01.MIB.0000191609.20713.ea. PMID 16306778.
- ↑ Naser SA, Sagramsingh SR, Naser AS, Thanigachalam S (June 2014). "Mycobacterium avium subspecies paratuberculosis causes Crohn's disease in some inflammatory bowel disease patients". World Journal of Gastroenterology. 20 (23): 7403–15. doi:10.3748/wjg.v20.i23.7403. PMC 4064085. PMID 24966610.
- ↑ Glubb DM, Gearry RB, Barclay ML, Roberts RL, Pearson J, Keenan JI, et al. (June 2011). "NOD2 and ATG16L1 polymorphisms affect monocyte responses in Crohn's disease". World Journal of Gastroenterology. 17 (23): 2829–37. doi:10.3748/wjg.v17.i23.2829 (inactive May 21, 2020). PMC 3120942. PMID 21734790.
{{cite journal}}: CS1 maint: DOI inactive as of May 2020 (link) - ↑ Clancy R, Ren Z, Turton J, Pang G, Wettstein A (May 2007). "Molecular evidence for Mycobacterium avium subspecies paratuberculosis (MAP) in Crohn's disease correlates with enhanced TNF-alpha secretion". Digestive and Liver Disease. 39 (5): 445–51. doi:10.1016/j.dld.2006.12.006. PMID 17317344.
- ↑ Nakase H, Tamaki H, Matsuura M, Chiba T, Okazaki K (November 2011). "Involvement of mycobacterium avium subspecies paratuberculosis in TNF-α production from macrophage: possible link between MAP and immune response in Crohn's disease". Inflammatory Bowel Diseases. 17 (11): E140–2. doi:10.1002/ibd.21750. PMID 21990211.
- ↑ 96.0 96.1 Baumgart M, Dogan B, Rishniw M, Weitzman G, Bosworth B, Yantiss R, et al. (September 2007). "Culture independent analysis of ileal mucosa reveals a selective increase in invasive Escherichia coli of novel phylogeny relative to depletion of Clostridiales in Crohn's disease involving the ileum". The ISME Journal. 1 (5): 403–18. doi:10.1038/ismej.2007.52. PMID 18043660.
- ↑ Sasaki M, Sitaraman SV, Babbin BA, Gerner-Smidt P, Ribot EM, Garrett N, Alpern JA, Akyildiz A, Theiss AL, Nusrat A, Klapproth JM (October 2007). "Invasive Escherichia coli are a feature of Crohn's disease". Laboratory Investigation; A Journal of Technical Methods and Pathology. 87 (10): 1042–54. doi:10.1038/labinvest.3700661. PMID 17660846.
- ↑ Darfeuille-Michaud A, Boudeau J, Bulois P, Neut C, Glasser AL, Barnich N, Bringer MA, Swidsinski A, Beaugerie L, Colombel JF (August 2004). "High prevalence of adherent-invasive Escherichia coli associated with ileal mucosa in Crohn's disease". Gastroenterology. 127 (2): 412–21. doi:10.1053/j.gastro.2004.04.061. PMID 15300573.
- ↑ Crohn's disease-associated adherent-invasive Escherichia coli adhesion is enhanced by exposure to the ubiquitous dietary polysaccharide maltodextrin. PLOS ONE. 2012;7(12):e52132. doi:10.1371/journal.pone.0052132. PMID 23251695. Bibcode: 2012PLoSO...752132N.
- ↑ Martinez-Medina M, Naves P, Blanco J, Aldeguer X, Blanco JE, Blanco M, Ponte C, Soriano F, Darfeuille-Michaud A, Garcia-Gil LJ (September 2009). "Biofilm formation as a novel phenotypic feature of adherent-invasive Escherichia coli (AIEC)". BMC Microbiology. 9 (1): 202. doi:10.1186/1471-2180-9-202. PMC 2759958. PMID 19772580.
- ↑ Chargui A, Cesaro A, Mimouna S, Fareh M, Brest P, Naquet P, Darfeuille-Michaud A, Hébuterne X, Mograbi B, Vouret-Craviari V, Hofman P (2012). "Subversion of autophagy in adherent invasive Escherichia coli-infected neutrophils induces inflammation and cell death". PLOS ONE. 7 (12): e51727. Bibcode:2012PLoSO...751727C. doi:10.1371/journal.pone.0051727. PMC 3522719. PMID 23272151.
- ↑ Craven M, Egan CE, Dowd SE, McDonough SP, Dogan B, Denkers EY, Bowman D, Scherl EJ, Simpson KW (2012). "Inflammation drives dysbiosis and bacterial invasion in murine models of ileal Crohn's disease". PLOS ONE. 7 (7): e41594. Bibcode:2012PLoSO...741594C. doi:10.1371/journal.pone.0041594. PMC 3404971. PMID 22848538.
- ↑ Barnich N, Darfeuille-Michaud A (January 2007). "Adherent-invasive Escherichia coli and Crohn's disease". Current Opinion in Gastroenterology. 23 (1): 16–20. doi:10.1097/MOG.0b013e3280105a38. PMID 17133079.
- ↑ Cenac N, Andrews CN, Holzhausen M, Chapman K, Cottrell G, Andrade-Gordon P, Steinhoff M, Barbara G, Beck P, Bunnett NW, Sharkey KA, Ferraz JG, Shaffer E, Vergnolle N (March 2007). "Role for protease activity in visceral pain in irritable bowel syndrome". The Journal of Clinical Investigation. 117 (3): 636–47. doi:10.1172/JCI29255. PMC 1794118. PMID 17304351.
- ↑ Cenac N, Coelho AM, Nguyen C, Compton S, Andrade-Gordon P, MacNaughton WK, Wallace JL, Hollenberg MD, Bunnett NW, Garcia-Villar R, Bueno L, Vergnolle N (November 2002). "Induction of intestinal inflammation in mouse by activation of proteinase-activated receptor-2". The American Journal of Pathology. 161 (5): 1903–15. doi:10.1016/S0002-9440(10)64466-5. PMC 1850779. PMID 12414536.[permanent dead link]
- ↑ Boorom KF, Smith H, Nimri L, Viscogliosi E, Spanakos G, Parkar U, Li LH, Zhou XN, Ok UZ, Leelayoova S, Jones MS (October 2008). "Oh my aching gut: irritable bowel syndrome, Blastocystis, and asymptomatic infection". Parasites & Vectors. 1 (1): 40. doi:10.1186/1756-3305-1-40. PMC 2627840. PMID 18937874.
- ↑ Hugot JP, Alberti C, Berrebi D, Bingen E, Cézard JP (December 2003). "Crohn's disease: the cold chain hypothesis". Lancet. 362 (9400): 2012–5. doi:10.1016/S0140-6736(03)15024-6. PMID 14683664.
- ↑ "Fridges blamed for Crohn's disease rise". Medical News Today. December 12, 2003. Archived from the original on January 3, 2009.
- ↑ Forbes A, Kalantzis T (July 2006). "Crohn's disease: the cold chain hypothesis". International Journal of Colorectal Disease. 21 (5): 399–401. doi:10.1007/s00384-005-0003-7. PMID 16059694.
- ↑ Subramanian S, Roberts CL, Hart CA, Martin HM, Edwards SW, Rhodes JM, Campbell BJ (February 2008). "Replication of Colonic Crohn's Disease Mucosal Escherichia coli Isolates within Macrophages and Their Susceptibility to Antibiotics". Antimicrobial Agents and Chemotherapy. 52 (2): 427–34. doi:10.1128/AAC.00375-07. PMC 2224732. PMID 18070962.
- ↑ Mpofu CM, Campbell BJ, Subramanian S, Marshall-Clarke S, Hart CA, Cross A, Roberts CL, McGoldrick A, Edwards SW, Rhodes JM (November 2007). "Microbial mannan inhibits bacterial killing by macrophages: a possible pathogenic mechanism for Crohn's disease". Gastroenterology. 133 (5): 1487–98. doi:10.1053/j.gastro.2007.08.004. PMID 17919633.
- ↑ "New insights into Crohn's Disease". Archived from the original on September 23, 2013.
- ↑ "Possible links between Crohn's disease and Paratuberculosis" (PDF). European Commission Directorate-General Health & Consumer Protection. Archived from the original (PDF) on December 17, 2008. Retrieved November 7, 2009.
- ↑ Gui GP, Thomas PR, Tizard ML, Lake J, Sanderson JD, Hermon-Taylor J (March 1997). "Two-year-outcomes analysis of Crohn's disease treated with rifabutin and macrolide antibiotics". The Journal of Antimicrobial Chemotherapy. 39 (3): 393–400. doi:10.1093/jac/39.3.393. PMID 9096189.
- ↑ Kumamoto CA (August 2011). "Inflammation and gastrointestinal Candida colonization". Current Opinion in Microbiology. 14 (4): 386–91. doi:10.1016/j.mib.2011.07.015. PMC 3163673. PMID 21802979.
- ↑ 116.0 116.1 Shoda R, Matsueda K, Yamato S, Umeda N (May 1996). "Epidemiologic analysis of Crohn disease in Japan: increased dietary intake of n-6 polyunsaturated fatty acids and animal protein relates to the increased incidence of Crohn disease in Japan". The American Journal of Clinical Nutrition. 63 (5): 741–5. doi:10.1093/ajcn/63.5.741. PMID 8615358.
- ↑ Lesko SM, Kaufman DW, Rosenberg L, Helmrich SP, Miller DR, Stolley PD, Shapiro S (November 1985). "Evidence for an increased risk of Crohn's disease in oral contraceptive users". Gastroenterology. 89 (5): 1046–9. doi:10.1016/0016-5085(85)90207-0. PMID 4043662.
- ↑ Reddy D, Siegel CA, Sands BE, Kane S (July 2006). "Possible association between isotretinoin and inflammatory bowel disease". The American Journal of Gastroenterology. 101 (7): 1569–73. PMID 16863562.
- ↑ Borobio E, Arín A, Valcayo A, Iñarrairaegui M, Nantes O, Prieto C (2004). "[Isotretinoin and ulcerous colitis]". Anales del Sistema Sanitario de Navarra (in Spanish). 27 (2): 241–3. doi:10.4321/S1137-66272004000300009. PMID 15381956.
{{cite journal}}: CS1 maint: unrecognized language (link) - ↑ Reniers DE, Howard JM (October 2001). "Isotretinoin-induced inflammatory bowel disease in an adolescent". The Annals of Pharmacotherapy. 35 (10): 1214–6. doi:10.1345/aph.10368. PMID 11675849. Archived from the original on June 29, 2012.
- ↑ Lomer MC, Hutchinson C, Volkert S, Greenfield SM, Catterall A, Thompson RP, Powell JJ (December 2004). "Dietary sources of inorganic microparticles and their intake in healthy subjects and patients with Crohn's disease". The British Journal of Nutrition. 92 (6): 947–55. doi:10.1079/bjn20041276. PMID 15613257.
- ↑ Powell JJ, Thoree V, Pele LC (October 2007). "Dietary microparticles and their impact on tolerance and immune responsiveness of the gastrointestinal tract". The British Journal of Nutrition. 98 Suppl 1: S59–63. doi:10.1017/S0007114507832922. PMC 2737314. PMID 17922962.
- ↑ Lee, T. W.; Russell, L.; Deng, M.; Gibson, P. R. (2013). "Association of doxycycline use with the development of gastroenteritis, irritable bowel syndrome and inflammatory bowel disease in Australians deployed abroad". Internal Medicine Journal. 43 (8): 919–926. doi:10.1111/imj.12179. ISSN 1445-5994. Archived from the original on July 30, 2020. Retrieved August 7, 2020.
- ↑ 124.0 124.1 Dj, Margolis; M, Fanelli; O, Hoffstad; Jd, Lewis (2010). "Potential Association Between the Oral Tetracycline Class of Antimicrobials Used to Treat Acne and Inflammatory Bowel Disease". The American journal of gastroenterology. PMID 20700115. Archived from the original on August 31, 2020. Retrieved June 7, 2020.
- ↑ Garrett, Jackie P-D.; Margolis, David J. (March 1, 2012). "Impact of Long-Term Antibiotic Use for Acne on Bacterial Ecology and Health Outcomes: A Review of Observational Studies". Current Dermatology Reports. 1 (1): 23–28. doi:10.1007/s13671-011-0001-7. ISSN 2162-4933. Archived from the original on August 28, 2021. Retrieved August 7, 2020.
- ↑ Elson CO, Cong Y, Weaver CT, Schoeb TR, McClanahan TK, Fick RB, Kastelein RA (2007). "Monoclonal anti-interleukin 23 reverses active colitis in a T cell-mediated model in mice". Gastroenterology. 132 (7): 2359–70. doi:10.1053/j.gastro.2007.03.104. PMID 17570211.
- ↑ Crawford JM. "The Gastrointestinal tract, Chapter 17". In Cotran RS, Kumar V, Robbins SL. Robbins Pathologic Basis of Disease: 5th Edition. W.B. Saunders and Company, Philadelphia, 1994.
- ↑ [1]
- ↑ HCP: Pill Cam, Capsule Endoscopy, Esophageal Endoscopy Archived June 16, 2008, at the Wayback Machine
- ↑ Scheinfeld NS, Teplitz E, McClain SA (November 2001). "Crohn's disease and lichen nitidus: a case report and comparison of common histopathologic features". Inflammatory Bowel Diseases. 7 (4): 314–8. doi:10.1097/00054725-200111000-00006. PMID 11720321.
- ↑ Tan WC, Allan RN (October 1993). "Diffuse jejunoileitis of Crohn's disease". Gut. 34 (10): 1374–8. doi:10.1136/gut.34.10.1374. PMC 1374544. PMID 8244104.
- ↑ 132.0 132.1 Gasche C, Scholmerich J, Brynskov J, D'Haens G, Hanauer SB, Irvine EJ, Jewell DP, Rachmilewitz D, Sachar DB, Sandborn WJ, Sutherland LR (February 2000). "A simple classification of Crohn's disease: report of the Working Party for the World Congresses of Gastroenterology, Vienna 1998". Inflammatory Bowel Diseases. 6 (1): 8–15. doi:10.1002/ibd.3780060103. PMID 10701144.
- ↑ Dubinsky MC, Fleshner PP (June 2003). "Treatment of Crohn's Disease of Inflammatory, Stenotic, and Fistulizing Phenotypes". Current Treatment Options in Gastroenterology. 6 (3): 183–200. doi:10.1007/s11938-003-0001-1. PMID 12744819.
- ↑ 134.0 134.1 Hara AK, Leighton JA, Heigh RI, Sharma VK, Silva AC, De Petris G, Hentz JG, Fleischer DE (January 2006). "Crohn disease of the small bowel: preliminary comparison among CT enterography, capsule endoscopy, small-bowel follow-through, and ileoscopy". Radiology. 238 (1): 128–34. doi:10.1148/radiol.2381050296. PMID 16373764.
- ↑ Triester SL, Leighton JA, Leontiadis GI, Gurudu SR, Fleischer DE, Hara AK, Heigh RI, Shiff AD, Sharma VK (May 2006). "A meta-analysis of the yield of capsule endoscopy compared to other diagnostic modalities in patients with non-stricturing small bowel Crohn's disease". The American Journal of Gastroenterology. 101 (5): 954–64. PMID 16696781.
- ↑ Dixon PM, Roulston ME, Nolan DJ (January 1993). "The small bowel enema: a ten year review". Clinical Radiology. 47 (1): 46–8. doi:10.1016/S0009-9260(05)81213-9. PMID 8428417.
- ↑ Carucci LR, Levine MS (March 2002). "Radiographic imaging of inflammatory bowel disease". Gastroenterology Clinics of North America. 31 (1): 93–117, ix. doi:10.1016/S0889-8553(01)00007-3. PMID 12122746.
- ↑ Rajesh A, Maglinte DD (January 2006). "Multislice CT enteroclysis: technique and clinical applications". Clinical Radiology. 61 (1): 31–9. doi:10.1016/j.crad.2005.08.006. PMID 16356814.
- ↑ Zissin R, Hertz M, Osadchy A, Novis B, Gayer G (February 2005). "Computed tomographic findings of abdominal complications of Crohn's disease--pictorial essay" (PDF). Canadian Association of Radiologists Journal. 56 (1): 25–35. PMID 15835588. Archived from the original (PDF) on April 6, 2008.
- ↑ Mackalski BA, Bernstein CN (May 2006). "New diagnostic imaging tools for inflammatory bowel disease". Gut. 55 (5): 733–41. doi:10.1136/gut.2005.076612. PMC 1856109. PMID 16609136.
- ↑ Sinha R, Rajiah P, Murphy P, Hawker P, Sanders S (October 2009). "Utility of high-resolution MR imaging in demonstrating transmural pathologic changes in Crohn disease". Radiographics. 29 (6): 1847–67. doi:10.1148/rg.296095503. PMID 19959525.
- ↑ Sinha R, Rajiah P, Ramachandran I, Sanders S, Murphy PD (May 2013). "Diffusion-weighted MR imaging of the gastrointestinal tract: technique, indications, and imaging findings". Radiographics. 33 (3): 655–76, discussion 676–80. doi:10.1148/rg.333125042. PMID 23674768.
- ↑ Goh J, O'Morain CA (February 2003). "Review article: nutrition and adult inflammatory bowel disease". Alimentary Pharmacology & Therapeutics. 17 (3): 307–20. doi:10.1046/j.1365-2036.2003.01482.x. PMID 12562443.
- ↑ Chamouard P, Richert Z, Meyer N, Rahmi G, Baumann R (July 2006). "Diagnostic value of C-reactive protein for predicting activity level of Crohn's disease". Clinical Gastroenterology and Hepatology. 4 (7): 882–7. doi:10.1016/j.cgh.2006.02.003. PMID 16630759.
- ↑ Kaila B, Orr K, Bernstein CN (December 2005). "The anti-Saccharomyces cerevisiae antibody assay in a province-wide practice: accurate in identifying cases of Crohn's disease and predicting inflammatory disease". Canadian Journal of Gastroenterology. 19 (12): 717–21. doi:10.1155/2005/147681. PMID 16341311.
- ↑ Israeli E, Grotto I, Gilburd B, Balicer RD, Goldin E, Wiik A, Shoenfeld Y (September 2005). "Anti-Saccharomyces cerevisiae and antineutrophil cytoplasmic antibodies as predictors of inflammatory bowel disease". Gut. 54 (9): 1232–6. doi:10.1136/gut.2004.060228. PMC 1774672. PMID 16099791.
- ↑ Ferrante M, Henckaerts L, Joossens M, Pierik M, Joossens S, Dotan N, Norman GL, Altstock RT, Van Steen K, Rutgeerts P, Van Assche G, Vermeire S (October 2007). "New serological markers in inflammatory bowel disease are associated with complicated disease behaviour". Gut. 56 (10): 1394–403. doi:10.1136/gut.2006.108043. PMC 2000264. PMID 17456509.
- ↑ Papp M, Altorjay I, Dotan N, Palatka K, Foldi I, Tumpek J, et al. (March 2008). "New serological markers for inflammatory bowel disease are associated with earlier age at onset, complicated disease behavior, risk for surgery, and NOD2/CARD15 genotype in a Hungarian IBD cohort". The American Journal of Gastroenterology. 103 (3): 665–81. PMID 18047543.
- ↑ Seow CH, Stempak JM, Xu W, Lan H, Griffiths AM, Greenberg GR, Steinhart AH, Dotan N, Silverberg MS (June 2009). "Novel anti-glycan antibodies related to inflammatory bowel disease diagnosis and phenotype". The American Journal of Gastroenterology. 104 (6): 1426–34. doi:10.1038/ajg.2009.79. PMID 19491856.
- ↑ Dotan I (December 2007). "Serologic markers in inflammatory bowel disease: tools for better diagnosis and disease stratification". Expert Review of Gastroenterology & Hepatology. 1 (2): 265–74. doi:10.1586/17474124.1.2.265. PMID 19072419.
- ↑ 151.0 151.1 Del Pinto R, Pietropaoli D, Chandar AK, Ferri C, Cominelli F (November 2015). "Association Between Inflammatory Bowel Disease and Vitamin D Deficiency: A Systematic Review and Meta-analysis". Inflammatory Bowel Diseases. 21 (11): 2708–17. doi:10.1097/MIB.0000000000000546. PMC 4615394. PMID 26348447.
- ↑ 152.0 152.1 Rubin, DT; Ananthakrishnan, AN; Siegel, CA; Sauer, BG; Long, MD (March 2019). "ACG Clinical Guideline: Ulcerative Colitis in Adults". The American journal of gastroenterology. 114 (3): 384–413. doi:10.14309/ajg.0000000000000152. PMID 30840605.
- ↑ Broomé U, Bergquist A (February 2006). "Primary sclerosing cholangitis, inflammatory bowel disease, and colon cancer". Seminars in Liver Disease. 26 (1): 31–41. doi:10.1055/s-2006-933561. PMID 16496231.
- ↑ Baumgart DC, Sandborn WJ (May 2007). "Inflammatory bowel disease: clinical aspects and established and evolving therapies". The Lancet. 369 (9573): 1641–57. doi:10.1016/S0140-6736(07)60751-X. PMID 17499606. Retrieved November 4, 2009.
- ↑ Shepherd NA (August 2002). "Granulomas in the diagnosis of intestinal Crohn's disease: a myth exploded?". Histopathology. 41 (2): 166–8. doi:10.1046/j.1365-2559.2002.01441.x. PMID 12147095.
- ↑ Mahadeva U, Martin JP, Patel NK, Price AB (July 2002). "Granulomatous ulcerative colitis: a re-appraisal of the mucosal granuloma in the distinction of Crohn's disease from ulcerative colitis". Histopathology. 41 (1): 50–5. doi:10.1046/j.1365-2559.2002.01416.x. PMID 12121237.
- ↑ DeRoche, TC; Xiao, SY; Liu, X (August 2014). "Histological evaluation in ulcerative colitis". Gastroenterology report. 2 (3): 178–92. doi:10.1093/gastro/gou031. PMID 24942757.
- ↑ Lewis NR, Scott BB (July 2006). "Systematic review: the use of serology to exclude or diagnose coeliac disease (a comparison of the endomysial and tissue transglutaminase antibody tests)". Alimentary Pharmacology & Therapeutics (Review). 24 (1): 47–54. doi:10.1111/j.1365-2036.2006.02967.x. PMID 16803602.
Both the endomysial antibody and tissue transglutaminase antibody have very high sensitivities (93% for both) and specificities (>99% and >98% respectively) for the diagnosis of typical coeliac disease with villous atrophy. (...) As the detection of at least partial villous atrophy was used to make a diagnosis of coeliac disease in the vast majority of studies, we can't assume that the same LRs apply to coeliac patients with lesser abnormality such as an increase in intraepithelial lymphocytes or electron-microscopic changes only. In fact, if such lesser abnormalities were used as criteria for diagnosing (and excluding) coeliac disease, the sensitivity of the tests could be lower (i.e. more false negatives), especially since a number of studies suggest that the EMA and tTG antibody tests are less sensitive with lesser degrees of mucosal abnormality
- ↑ Rodrigo L, Garrote JA, Vivas S (September 2008). "[Celiac disease]". Medicina Clinica (Review) (in spanish). 131 (7): 264–70. doi:10.1016/S0025-7753(08)72247-4. PMID 18775218. Archived from the original on March 19, 2016.
Estos marcadores presentan en general una elevada sensibilidad y especificidad (cercanas al 90%) en presencia de atrofia marcada de las vellosidades intestinales. Sin embargo, muestran una notable disminución de la sensibilidad (del orden del 40-50%) en casos con atrofia vellositaria leve o cambios mínimos. These markers generally have high sensitivity and specificity (around 90%) in the presence of marked atrophy of the villi. However, they show a marked decrease in sensitivity (of the order of 40-50%) in cases with mild villous atrophy or minimal changes.
{{cite journal}}: CS1 maint: unrecognized language (link) - ↑ Rostami Nejad M, Hogg-Kollars S, Ishaq S, Rostami K (2011). "Subclinical celiac disease and gluten sensitivity". Gastroenterology and Hepatology from Bed to Bench (Review). 4 (3): 102–8. PMC 4017418. PMID 24834166.
- ↑ Bold J, Rostami K (2011). "Gluten tolerance; potential challenges in treatment strategies". Gastroenterology and Hepatology from Bed to Bench (Review). 4 (2): 53–7. PMC 4017406. PMID 24834157.
- ↑ 162.0 162.1 Agabegi, Elizabeth D; Agabegi, Steven S. (2008). "Inflammatory bowel disease (IBD)". Step-Up to Medicine (Step-Up Series). Hagerstwon, MD: Lippincott Williams & Wilkins. pp. 152–156. ISBN 0-7817-7153-6.
{{cite book}}: Unknown parameter|name-list-format=ignored (help) - ↑ Feller M, Huwiler K, Schoepfer A, Shang A, Furrer H, Egger M (February 2010). "Long-term antibiotic treatment for Crohn's disease: systematic review and meta-analysis of placebo-controlled trials". Clinical Infectious Diseases. 50 (4): 473–80. doi:10.1086/649923. PMID 20067425.
- ↑ Prantera C, Scribano ML (July 2009). "Antibiotics and probiotics in inflammatory bowel disease: why, when, and how". Current Opinion in Gastroenterology. 25 (4): 329–33. doi:10.1097/MOG.0b013e32832b20bf. PMID 19444096.
- ↑ 165.0 165.1 165.2 Fries WS, Nazario B (May 16, 2007). "Crohn's Disease: 54 Tips to Help You Manage". WebMD. Archived from the original on February 8, 2008. Retrieved February 14, 2008.
- ↑ Hou JK, Abraham B, El-Serag H (April 2011). "Dietary intake and risk of developing inflammatory bowel disease: a systematic review of the literature" (PDF). The American Journal of Gastroenterology. 106 (4): 563–73. doi:10.1038/ajg.2011.44. PMID 21468064. Archived from the original (PDF) on June 12, 2015.
- ↑ Nutrition and Diagnosis-Related Care, 7th edition. Baltimore, MD: Lippincott Williams & Wilkins; 2008. ISBN 978-1-60831-017-3. p. 1020 (pp 431).
- ↑ Shanahan F (January 2002). "Crohn's disease". Lancet. 359 (9300): 62–9. doi:10.1016/S0140-6736(02)07284-7. PMID 11809204.
- ↑ "FDA Approves Cimzia to Treat Crohn's Disease" (Press release). Food and Drug Administration (FDA). April 22, 2008. Archived from the original on October 20, 2009. Retrieved November 5, 2009.
- ↑ "Prescribing information ustekinumab" (PDF). FDA. Archived (PDF) from the original on October 18, 2020. Retrieved May 23, 2019.
- ↑ Sandborn WJ, Colombel JF, Enns R, Feagan BG, Hanauer SB, Lawrance IC, Panaccione R, Sanders M, Schreiber S, Targan S, van Deventer S, Goldblum R, Despain D, Hogge GS, Rutgeerts P, et al. (International Efficacy of Natalizumab as Active Crohn's Therapy (ENACT-1) Trial Group; Evaluation of Natalizumab as Continuous Therapy (ENACT-2) Trial Group) (November 2005). "Natalizumab induction and maintenance therapy for Crohn's disease". The New England Journal of Medicine. 353 (18): 1912–25. doi:10.1056/NEJMoa043335. PMID 16267322.
- ↑ Natalizumab for induction of remission in Crohn's disease. The Cochrane Database of Systematic Reviews. 2018;8:CD006097. doi:10.1002/14651858.CD006097.pub3. PMID 30068022.
- ↑ Oxford Handbook of Clinical Medicine. 7th ed. Oxford University Press; 2007. ISBN 978-0-19-856837-7. p. 266–7.
- ↑ 174.0 174.1 174.2 Anti-IL-12/23p40 antibodies for induction of remission in Crohn's disease. The Cochrane Database of Systematic Reviews. 2016;11:CD007572. doi:10.1002/14651858.CD007572.pub3. PMID 27885650.
- ↑ Mowat C, Cole A, Windsor A, Ahmad T, Arnott I, Driscoll R, Mitton S, Orchard T, Rutter M, Younge L, Lees C, Ho GT, Satsangi J, Bloom S, et al. (IBD Section of the British Society of Gastroenterology) (May 2011). "Guidelines for the management of inflammatory bowel disease in adults". Gut. 60 (5): 571–607. doi:10.1136/gut.2010.224154. PMID 21464096.
- ↑ 176.0 176.1 176.2 Goddard AF, James MW, McIntyre AS, Scott BB (October 2011). "Guidelines for the management of iron deficiency anaemia". Gut. 60 (10): 1309–16. doi:10.1136/gut.2010.228874. PMID 21561874.
- ↑ 177.0 177.1 Inflamm Bowel Dis Volume 13, Number 12, December 2007
- ↑ Kristo I, Stift A, Bergmann M, Riss S (May 2015). "Surgical recurrence in Crohn's disease: Are we getting better?". World Journal of Gastroenterology. 21 (20): 6097–100. doi:10.3748/wjg.v21.i20.6097. PMC 4445088. PMID 26034346.
- ↑ Tresca AJ (January 12, 2007). "Resection Surgery for Crohn's Disease". About.com. Archived from the original on November 13, 2007. Retrieved February 14, 2008.
{{cite web}}: CS1 maint: uses authors parameter (link) - ↑ Ozuner G, Fazio VW, Lavery IC, Milsom JW, Strong SA (November 1996). "Reoperative rates for Crohn's disease following strictureplasty. Long-term analysis". Diseases of the Colon and Rectum. 39 (11): 1199–203. doi:10.1007/BF02055108. PMID 8918424.
- ↑ Rutgeerts P, Geboes K, Vantrappen G, Beyls J, Kerremans R, Hiele M (October 1990). "Predictability of the postoperative course of Crohn's disease". Gastroenterology. 99 (4): 956–63. doi:10.1016/0016-5085(90)90613-6. PMID 2394349.
- ↑ Yamamoto T, Bamba T, Umegae S, Matsumoto K (August 2013). "The impact of early endoscopic lesions on the clinical course of patients following ileocolonic resection for Crohn's disease: A 5-year prospective cohort study". United European Gastroenterology Journal. 1 (4): 294–8. doi:10.1177/2050640613495197. PMC 4040796. PMID 24917974.
- ↑ "Short Bowel Syndrome". National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). July 2015. Archived from the original on December 9, 2019. Retrieved December 8, 2019.
- ↑ Everyday Health. Intestinal transplant for Crohn's disease; October 24, 2006 [archived October 8, 2008].
- ↑ Hofmann AF (April 1967). "The syndrome of ileal disease and the broken enterohepatic circulation: cholerheic enteropathy". Gastroenterology. 52 (4): 752–7. doi:10.1016/S0016-5085(67)80140-9. PMID 5337211.
- ↑ 186.0 186.1 Szigethy E, McLafferty L, Goyal A (April 2010). "Inflammatory bowel disease". Child and Adolescent Psychiatric Clinics of North America (Submitted manuscript). 19 (2): 301–18, ix. doi:10.1016/j.chc.2010.01.007. PMID 20478501. Archived from the original on April 28, 2021. Retrieved August 7, 2020.
- ↑ Ballou S, Keefer L (January 2017). "Psychological Interventions for Irritable Bowel Syndrome and Inflammatory Bowel Diseases". Clinical and Translational Gastroenterology. 8 (1): e214. doi:10.1038/ctg.2016.69. PMC 5288603. PMID 28102860.
- ↑ 188.0 188.1 Caprilli R, Gassull MA, Escher JC, Moser G, Munkholm P, Forbes A, et al. (European Crohn's Colitis Organisation) (March 2006). "European evidence based consensus on the diagnosis and management of Crohn's disease: special situations". Gut. 55 Suppl 1 (Suppl 1): i36–58. doi:10.1136/gut.2005.081950c. PMC 1859996. PMID 16481630.
the colitis activity index fell significantly in the treatment group compared to the sham acupuncture group. However, recruitment did not reach its target and the number of patients was small.
- ↑ Joos S, Brinkhaus B, Maluche C, Maupai N, Kohnen R, Kraehmer N, Hahn EG, Schuppan D (2004). "Acupuncture and moxibustion in the treatment of active Crohn's disease: a randomized controlled study". Digestion. 69 (3): 131–9. doi:10.1159/000078151. PMID 15114043.
- ↑ Joos S (June 2011). "Review on efficacy and health services research studies of complementary and alternative medicine in inflammatory bowel disease". Chinese Journal of Integrative Medicine. 17 (6): 403–9. doi:10.1007/s11655-011-0758-3. PMID 21660673.
- ↑ Smith K (2012). "Homeopathy is Unscientific and Unethical". Bioethics. 26 (9): 508–512. doi:10.1111/j.1467-8519.2011.01956.x. Archived from the original on October 29, 2017. Retrieved August 7, 2020.
- ↑ 192.0 192.1 Baran GR, Kiana MF, Samuel SP (2014). "Science, Pseudoscience, and Not Science: How do They Differ?". Chapter 2: Science, Pseudoscience, and Not Science: How Do They Differ?. Healthcare and Biomedical Technology in the 21st Century. Springer. pp. 19–57. doi:10.1007/978-1-4614-8541-4_2. ISBN 978-1-4614-8540-7.
within the traditional medical community it is considered to be quackery
- ↑ 193.0 193.1 Ladyman J (2013). "Chapter 3: Towards a Demarcation of Science from Pseudoscience". In Pigliucci M, Boudry M (eds.). Philosophy of Pseudoscience: Reconsidering the Demarcation Problem. University of Chicago Press. pp. 48–49. ISBN 978-0-226-05196-3.
Yet homeopathy is a paradigmatic example of pseudoscience. It is neither simply bad science nor science fraud, but rather profoundly departs from scientific method and theories while being described as scientific by some of its adherents (often sincerely).
- ↑ Ernst E (December 2002). "A systematic review of systematic reviews of homeopathy". British Journal of Clinical Pharmacology. 54 (6): 577–82. doi:10.1046/j.1365-2125.2002.01699.x. PMC 1874503. PMID 12492603.
- ↑ Shang A, Huwiler-Müntener K, Nartey L, Jüni P, Dörig S, Sterne JA, Pewsner D, Egger M (2005). "Are the clinical effects of homoeopathy placebo effects? Comparative study of placebo-controlled trials of homoeopathy and allopathy". Lancet. 366 (9487): 726–32. doi:10.1016/S0140-6736(05)67177-2. PMID 16125589.
- ↑ "Evidence Check 2: Homeopathy - Science and Technology Committee". British House of Commons Science and Technology Committee. February 22, 2010. Archived from the original on September 19, 2015. Retrieved April 5, 2014.
- ↑ Naftali T, Mechulam R, Lev LB, Konikoff FM (2014). "Cannabis for inflammatory bowel disease". Digestive Diseases. 32 (4): 468–74. doi:10.1159/000358155. PMID 24969296.
- ↑ Cannabis for the treatment of Crohn's disease. The Cochrane Database of Systematic Reviews. 2018-11-08;11:CD012853. doi:10.1002/14651858.CD012853.pub2. PMID 30407616.
- ↑ "Crohn's disease - Prognosis". University of Maryland Medical Centre. Archived from the original on August 29, 2012. Retrieved October 19, 2012.
- ↑ 201.0 201.1 Hiatt RA, Kaufman L (November 1988). "Epidemiology of inflammatory bowel disease in a defined northern California population". The Western Journal of Medicine. 149 (5): 541–6. PMC 1026530. PMID 3250100.
- ↑ Moum B, Vatn MH, Ekbom A, Aadland E, Fausa O, Lygren I, Stray N, Sauar J, Schulz T (April 1996). "Incidence of Crohn's disease in four counties in southeastern Norway, 1990-93. A prospective population-based study. The Inflammatory Bowel South-Eastern Norway (IBSEN) Study Group of Gastroenterologists". Scandinavian Journal of Gastroenterology. 31 (4): 355–61. doi:10.3109/00365529609006410. PMID 8726303.
- ↑ Shivananda S, Lennard-Jones J, Logan R, Fear N, Price A, Carpenter L, van Blankenstein M (November 1996). "Incidence of inflammatory bowel disease across Europe: is there a difference between north and south? Results of the European Collaborative Study on Inflammatory Bowel Disease (EC-IBD)". Gut. 39 (5): 690–7. doi:10.1136/gut.39.5.690. PMC 1383393. PMID 9014768.
- ↑ Yang H, McElree C, Roth MP, Shanahan F, Targan SR, Rotter JI (April 1993). "Familial empirical risks for inflammatory bowel disease: differences between Jews and non-Jews". Gut. 34 (4): 517–24. doi:10.1136/gut.34.4.517. PMC 1374314. PMID 8491401.
- ↑ Seksik P, Nion-Larmurier I, Sokol H, Beaugerie L, Cosnes J (May 2009). "Effects of light smoking consumption on the clinical course of Crohn's disease". Inflammatory Bowel Diseases. 15 (5): 734–41. doi:10.1002/ibd.20828. PMID 19067428.
- ↑ "Crohn's disease manifests differently in boys and girls". Crohn's and Colitis Foundation of America. Archived from the original on February 16, 2008.
- ↑ "Who is affected by Crohn's disease". Healthwise. Archived from the original on January 23, 2009.
- ↑ Satsangi J, Jewell DP, Bell JI (May 1997). "The genetics of inflammatory bowel disease". Gut. 40 (5): 572–4. doi:10.1136/gut.40.5.572. PMC 1027155. PMID 9203931.
- ↑ Tysk C, Lindberg E, Järnerot G, Flodérus-Myrhed B (July 1988). "Ulcerative colitis and Crohn's disease in an unselected population of monozygotic and dizygotic twins. A study of heritability and the influence of smoking". Gut. 29 (7): 990–6. doi:10.1136/gut.29.7.990. PMC 1433769. PMID 3396969.
- ↑ Burisch J, Jess T, Martinato M, Lakatos PL (May 2013). "The burden of inflammatory bowel disease in Europe". Journal of Crohn's & Colitis. 7 (4): 322–37. doi:10.1016/j.crohns.2013.01.010. PMID 23395397.
- ↑ 211.0 211.1 Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EI, Panaccione R, Ghosh S, Wu JC, Chan FK, Sung JJ, Kaplan GG (December 2018). "Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies". Lancet. 390 (10114): 2769–2778. doi:10.1016/S0140-6736(17)32448-0. PMID 29050646.
- ↑ Kirsner JB (June 1988). "Historical aspects of inflammatory bowel disease". Journal of Clinical Gastroenterology. 10 (3): 286–97. doi:10.1097/00004836-198806000-00012. PMID 2980764.
- ↑ Lichtarowicz AM, Mayberry JF (August 1988). "Antoni Lésniowski and his contribution to regional enteritis (Crohn's disease)". Journal of the Royal Society of Medicine. 81 (8): 468–70. doi:10.1177/014107688808100817. PMC 1291720. PMID 3047387.
- ↑ Agrawal G, Borody TJ, Chamberlin W (2014). "'Global warming' to Mycobacterium avium subspecies paratuberculosis". Future Microbiology. 9 (7): 829–32. doi:10.2217/fmb.14.52. PMID 25156371.
- ↑ Scribano ML, Prantera C (February 2013). "Use of antibiotics in the treatment of Crohn's disease". World Journal of Gastroenterology. 19 (5): 648–53. doi:10.3748/wjg.v19.i5.648. PMC 3574590. PMID 23429474.
- ↑ Chamberlin W, Borody TJ, Campbell J (November 2011). "Primary treatment of Crohn's disease: combined antibiotics taking center stage". Expert Review of Clinical Immunology. 7 (6): 751–60. doi:10.1586/eci.11.43. PMID 22014016.
- ↑ Fundamentals of microbiology. Burlington, MA: Jones & Bartlett Learning; 2014. ISBN 9781449688615.
- ↑ Elliott DE, Weinstock JV (November 2012). "Where are we on worms?". Current Opinion in Gastroenterology. 28 (6): 551–6. doi:10.1097/MOG.0b013e3283572f73. PMC 3744105. PMID 23079675.
- ↑ Weinstock JV, Elliott DE (March 2013). "Translatability of helminth therapy in inflammatory bowel diseases". International Journal for Parasitology. 43 (3–4): 245–51. doi:10.1016/j.ijpara.2012.10.016. PMC 3683647. PMID 23178819.
Early clinical trials suggested that exposure to helminths such as Trichuris suis or Necator americanus can improve IBD.
- ↑ Coronado Biosciences Announces Top-Line Results From Its TRUST-I Phase 2 Clinical Trial of TSO for the Treatment of Crohn's Disease; 2013-10-14 [archived August 16, 2016]. en-US.
- ↑ Biosciences, Coronado (November 7, 2013). "Coronado Biosciences Announces Independent Data Monitoring Committee Recommendation to Discontinue Falk Phase 2 Trial of TSO in Crohn's Disease". Archived from the original on August 16, 2016. Retrieved August 16, 2016.
- ↑ Srinivasan R, Akobeng AK (April 2009). "Thalidomide and thalidomide analogues for induction of remission in Crohn's disease". The Cochrane Database of Systematic Reviews (2): CD007350. doi:10.1002/14651858.CD007350.pub2. PMID 19370684.
- ↑ Akobeng AK, Stokkers PC (April 2009). "Thalidomide and thalidomide analogues for maintenance of remission in Crohn's disease". The Cochrane Database of Systematic Reviews (2): CD007351. doi:10.1002/14651858.CD007351.pub2. PMC 7207562. PMID 19370685.
Further reading
- Lichtenstein GR, Loftus EV, Isaacs KL, Regueiro MD, Gerson LB, Sands BE (April 2018). "ACG Clinical Guideline: Management of Crohn's Disease in Adults". Am. J. Gastroenterol. 113 (4): 481–517. doi:10.1038/ajg.2018.27. PMID 29610508.
External links
- "Crohn's disease". MedlinePlus. U.S. National Library of Medicine. Archived from the original on July 28, 2020. Retrieved August 7, 2020.
| Classification | |
|---|---|
| External resources |
- Pages with script errors
- All articles with dead external links
- Articles with dead external links from December 2019
- Articles with invalid date parameter in template
- Articles with permanently dead external links
- CS1: long volume value
- Webarchive template wayback links
- CS1 maint: multiple names: authors list
- CS1 maint: DOI inactive as of May 2020
- Articles with dead external links from July 2017
- CS1 maint: unrecognized language
- CS1 errors: unsupported parameter
- CS1 maint: uses authors parameter
- Use mdy dates from July 2017
- All articles with unsourced statements
- Articles with unsourced statements from July 2020
- Articles with hatnote templates targeting a nonexistent page
- Articles containing potentially dated statements from 2017
- All articles containing potentially dated statements
- Articles containing Polish-language text
- Good articles
- Abdominal pain
- Articles containing video clips
- Autoimmune diseases
- Inflammations
- Membrane transport protein disorders
- Noninfective enteritis and colitis
- RTT
- RTTEM
- Steroid-responsive inflammatory conditions
- WHRTT