Tropone
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Cyclohepta-2,4,6-trien-1-one | |||
| Other names
Cyclohepta-2,4,6-trienone
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| ECHA InfoCard | 100.007.933 | ||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C7H6O | |||
| Molar mass | 106.12 g/mol | ||
| Density | 1.094 g/mL | ||
| Boiling point | 113 °C (235 °F; 386 K) (15 mmHg) | ||
| Hazards | |||
| Flash point | > 113 °C (235 °F; 386 K) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Tropone or 2,4,6-cycloheptatrien-1-one is an organic compound with some importance in organic chemistry as a non-benzenoid aromatic.[2] The compound consists of a ring of seven carbon atoms with three conjugated alkene groups and a ketone group. The related compound tropolone (2-hydroxy-2,4,6-cycloheptatrien-1-one) has an additional alcohol (or an enol including the double bond) group next to the ketone. Tropones are uncommon in natural products, with the notable exception of the 2-hydroxyl derivatives, which are called tropolones.
Tropone has been known since 1951 and is also called cycloheptatrienylium oxide. The name tropolone was coined by M. J. S. Dewar in 1945 in connection to perceived aromatic properties.[3]
Properties
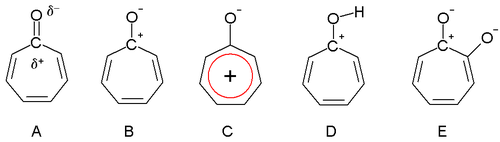
Dewar in 1945 proposed that tropones could have aromatic properties. The carbonyl group is more polarized as a result of the triene ring, giving a partial positive charge on the carbon atom (A) and a partial negative charge on oxygen. In an extreme case, the carbon atom has a full positive charge (B) forming a tropylium ion ring which is an aromatic 6 electron system (C).
Tropones are also basic (D) as a result of the aromatic stabilization. This property can be observed in the ease of salt formation with acids. The dipole moment for tropone is 4.17 D compared to a value of only 3.04 D for cycloheptanone. This difference is consistent with stabilization of the dipolar resonance structure.
Synthesis
Numerous methods exist for the organic synthesis of tropones and its derivatives. Two selected methods for the synthesis of tropone are by selenium dioxide oxidation of cycloheptatriene[4] and indirectly from tropinone by a Hofmann elimination and a bromination.[2]
Reactions
Tropone undergoes ring contraction to benzoic acid with potassium hydroxide at elevated temperature. Many derivatives also contract to the corresponding arenes.[2] Tropone reacts in electrophilic substitution, for instance with bromine, but the reaction proceeds through the 1,2-addition product and is not an electrophilic aromatic substitution.[2]
Tropone derivatives also react in nucleophilic substitution very much like in nucleophilic aromatic substitution.[2]
Tropone is also found to react in an [8+3]annulation with a cinnamic aldehyde[5]
Diene character
Tropone behaves as a diene in a Diels-Alder reactions, for instance with maleic anhydride.[2] Similarly, it forms adducts with iron tricarbonyl, akin to (butadiene)iron tricarbonyl.[6]
Derivatives
| Name | Chemical structure | Natural sources |
|---|---|---|
| Tropolone |  |
Pseudomonas lindbergii, Pseudomonas plantarii[7] |
| Hinokitiol |  |
Cupressaceae trees[8] |
| Stipitatic acid |  |
Talaromyces stipitatus[9] |
| Tropodithietic acid |  |
Phaeobacter piscinae, Phaeobacter inhibens, Phaeobacter gallaeciensis[10][11] |
| Colchicine |  |
Colchicum autumnale, Gloriosa superba[12] |
Other tropone derivatives include puberulonic and puberulic acids, roseobacticides, pernambucone, crototropone, orobanone.[13][14][15][16][17]
References
- ^ Tropone at Sigma-Aldrich
- ^ a b c d e f Pauson, Peter L. (1955). "Tropones and Tropolones". Chem. Rev. 55 (1): 9–136. doi:10.1021/cr50001a002.
- ^ M. J. S. Dewar (1945). "Structure of Stipitatic Acid". Nature. 155 (3924): 50–51. Bibcode:1945Natur.155...50D. doi:10.1038/155050b0. S2CID 4086209.
- ^ Dahnke, Karl R.; Paquette, Leo A. (1993). "Inverse Electron-Demand Diels-Alder Cycloaddition of a Ketene Dithioacetal. Copper Hydride-Promoted Reduction of a Conjugated Enone. 9-Dithiolanobicyclo[3.2.2]non-6-en-2-one". Org. Synth. 71: 181. doi:10.15227/orgsyn.071.0181.
- ^ An N-Heterocyclic Carbene-Catalyzed [8 + 3] Annulation of Tropone and Enals via Homoenolate Vijay Nair, Manojkumar Poonoth, Sreekumar Vellalath, Eringathodi Suresh, and Rajasekaran Thirumalai J. Org. Chem.; 2006; 71(23) pp 8964 - 8965; (Note) doi:10.1021/jo0615706
- ^ Dodge, R. P. (1964). "The Crystal and Molecular Structure of Tropone Iron Tricarbonyl". Journal of the American Chemical Society. 86 (24): 5429–5431. doi:10.1021/ja01078a013.
- ^ Liu, Na; Song, Wangze; Schienebeck, Casi M.; Zhang, Min; Tang, Weiping (December 2014). "Synthesis of naturally occurring tropones and tropolones". Tetrahedron. 70 (49): 9281–9305. doi:10.1016/j.tet.2014.07.065. PMC 4228802. PMID 25400298.
- ^ Saniewski, Marian; Horbowicz, Marcin; Kanlayanarat, Sirichai (10 September 2014). "The Biological Activities of Troponoids and Their Use in Agriculture A Review". Journal of Horticultural Research. 22 (1): 5–19. doi:10.2478/johr-2014-0001.
- ^ Davison, J.; al Fahad, A.; Cai, M.; Song, Z.; Yehia, S. Y.; Lazarus, C. M.; Bailey, A. M.; Simpson, T. J.; Cox, R. J. (15 May 2012). "Genetic, molecular, and biochemical basis of fungal tropolone biosynthesis". Proceedings of the National Academy of Sciences. 109 (20): 7642–7647. doi:10.1073/pnas.1201469109. PMC 3356636. PMID 22508998.
- ^ Rabe, Patrick; Klapschinski, Tim A; Brock, Nelson L; Citron, Christian A; D’Alvise, Paul; Gram, Lone; Dickschat, Jeroen S (6 August 2014). "Synthesis and bioactivity of analogues of the marine antibiotic tropodithietic acid". Beilstein Journal of Organic Chemistry. 10: 1796–1801. doi:10.3762/bjoc.10.188. PMC 4142847. PMID 25161739.
- ^ Beyersmann, Paul G.; Tomasch, Jürgen; Son, Kwangmin; Stocker, Roman; Göker, Markus; Wagner-Döbler, Irene; Simon, Meinhard; Brinkhoff, Thorsten (December 2017). "Dual function of tropodithietic acid as antibiotic and signaling molecule in global gene regulation of the probiotic bacterium Phaeobacter inhibens". Scientific Reports. 7 (1): 730. Bibcode:2017NatSR...7..730B. doi:10.1038/s41598-017-00784-7. PMC 5429656. PMID 28389641.
- ^ Keith, Michael P.; Gilliland, William R.; Uhl, Kathleen (2009). "GOUT". Pharmacology and Therapeutics: 1039–1046. doi:10.1016/B978-1-4160-3291-5.50079-2. ISBN 978-1-4160-3291-5.
- ^ Thiel, Verena; Brinkhoff, Thorsten; Dickschat, Jeroen S.; Wickel, Susanne; Grunenberg, Jörg; Wagner-Döbler, Irene; Simon, Meinhard; Schulz, Stefan (10 December 2009). "Identification and biosynthesis of tropone derivatives and sulfur volatiles produced by bacteria of the marine Roseobacter clade". Organic & Biomolecular Chemistry. 8 (1): 234–246. doi:10.1039/B909133E. PMID 20024154.
- ^ Duan, Ying; Petzold, Melanie; Saleem-Batcha, Raspudin; Teufel, Robin (September 2020). "Bacterial Tropone Natural Products and Derivatives: Overview of their Biosynthesis, Bioactivities, Ecological Role and Biotechnological Potential". ChemBioChem. 21 (17): 2384–2407. doi:10.1002/cbic.201900786. PMC 7497051. PMID 32239689.
- ^ Randau, K. P.; Sproll, S.; Lerche, H.; Bracher, F. (1 May 2009). "Pernambucone, a new tropone derivative from Croton argyroglossum". Die Pharmazie. 64 (5): 350–351. doi:10.1691/ph.2009.7592. PMID 19530449.
- ^ Bracher, Franz; Randau, Karina P.; Lerche, Holger (1 April 2008). "Crototropone, a new tropone derivative from Croton zehntneri". Fitoterapia. 79 (3): 236–237. doi:10.1016/j.fitote.2007.12.001. PMID 18321658.
- ^ Fruchier, Alain; Rascol, Jean-Pierre; Andary, Claude; Privatt, Guy (1 January 1981). "A tropone derivative from orobanche rapum-genistae". Phytochemistry. 20 (4): 777–779. Bibcode:1981PChem..20..777F. doi:10.1016/0031-9422(81)85173-4.
- Articles without EBI source
- Articles without KEGG source
- ECHA InfoCard ID from Wikidata
- Articles containing unverified chemical infoboxes
- Chembox image size set
- Articles with short description
- Short description matches Wikidata
- Articles with GND identifiers
- Tropones
- Simple aromatic rings
- Non-benzenoid aromatic carbocycles