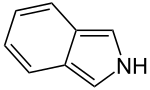
Isoindole
| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
2H-Isoindole[1] | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C8H7N | |
| Molar mass | 117.15 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
In organic chemistry and heterocyclic chemistry, isoindole consists of a benzene ring fused with pyrrole.[2] The compound is an isomer of indole. Its reduced form is isoindoline. The parent isoindole is a rarely encountered in the technical literature, but substituted derivatives are useful commercially and occur naturally. Isoindoles units occur in phthalocyanines, an important family of dyes. Some alkaloids containing isoindole have been isolated and characterized.[3][4]
Synthesis
The parent isoindole was prepared by flash vacuum pyrolysis of an N-substituted isoindoline.[5] N-Substituted isoindoles, which are easier to handle, can be prepared by dehydration of isoindoline-N-oxides. They also arise by myriad other methods, e.g., starting from xylylene dibromide (C6H4(CH2Br)2).
Structure and tautomerism of 2-H-isoindoles
Unlike indole, isoindoles exhibit noticeable alternation in the C-C bond lengths, which is consistent with their description as pyrrole derivatives fused to a butadiene.
In solution, the 2H-isoindole tautomer predominates. It resembles a pyrrole more than a simple imine.[6] The degree to which the 2H predominates depends on the solvent, and can vary with the substituent in substituted isoindoles.[7]
N-Substituted isoindoles do not engage is tautomerism and are therefore simpler to study.
The commercially important phthalimide is an isoindole-1,3-dione with two carbonyl groups attached to the heterocyclic ring.
- Illustrative Isoindoline Derivatives
-
Pigment yellow 139, a common high performance pigment.
-
Pigment yellow 185, a common high performance pigment.
-
Copper phthalocyanine, one of the most pervasive synthetic pigments.
See also
- 1,3-Disubstituted Isoindolines.
- Isoindene with nitrogen replaced by a methylene group.
References
- ^ International Union of Pure and Applied Chemistry (2014). Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013. The Royal Society of Chemistry. p. 213. doi:10.1039/9781849733069. ISBN 978-0-85404-182-4.
- ^ Gilchrist, T. L. (1987). Heterocyclic Chemistry. Longman. ISBN 0-582-01422-0.
- ^ Heugebaert, Thomas S. A.; Roman, Bart I.; Stevens, Christian V. "Synthesis of isoindoles and related iso-condensed heteroaromatic pyrroles" Chemical Society Reviews 2012, volume 41, pp. 5626-5640. doi:10.1039/c2cs35093a
- ^ See for example: Zhang, X.; Ye, W.; Zhao, S.; Che, C. T. (2004). "Isoquinoline and isoindole alkaloids from Menispermum dauricum". Phytochemistry. 65 (7): 929–932. doi:10.1016/j.phytochem.2003.12.004. PMID 15081297.
- ^ R. Bonnett and R. F. C. Brown "Isoindole" J. Chem. Soc., Chem. Commun., 1972, 393-395. doi:10.1039/C39720000393
- ^ Alan R. Katritzky; Christopher A. Ramsden; J. Joule; Viktor V. Zhdankin (2010). Handbook of Heterocyclic Chemistry. Elsevier. p. 133.
- ^ John A. Joule; Keith Mills (2010). Heterocyclic Chemistry. John Wiley & Sons. p. 447.