Bictegravir/emtricitabine/tenofovir alafenamide
| Combination of | |
|---|---|
| Bictegravir | Integrase inhibitor |
| Emtricitabine | Nucleoside reverse transcriptase inhibitor |
| Tenofovir alafenamide | Nucleotide reverse transcriptase inhibitor |
| Names | |
| Trade names | Biktarvy |
| Clinical data | |
| Main uses | HIV/AIDS[1] |
| Side effects | Diarrhea, nausea, headache[1] |
| Pregnancy category | |
| Routes of use | By mouth |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a618012 |
| Legal | |
| License data | |
| Legal status | |
Bictegravir/emtricitabine/tenofovir alafenamide, sold under the brand name Biktarvy, is a combination medication used to treat HIV/AIDS.[1] It is taken by mouth as one tablet a day.[4] It is used in people who weight more than 14 kg.[1]
Common side effects include diarrhea, nausea, and headache.[1] Other side effects may include immune reconstitution syndrome, kidney problems, liver problems, and lactic acidosis.[1] Safety in pregnancy is unclear.[5] It is a combination of bictegravir, an integrase inhibitor; and emtricitabine and tenofovir alafenamide, both nucleoside reverse transcriptase inhibitor (NRTIs).[1]
The combination was approved for medical use in the United States and Europe in 2018.[1][6] In the United Kingdom a month of medication costs the NHS about £880 as of 2021.[4] This amount in the United States costs about 3,500 USD.[7]
Medical uses
This drug regimen is intended and approved for adults with HIV-1 infection who have had no previous antiretroviral treatment or for those with less than 50 copies of HIV-1 RNA per mL. Patients with HIV-1 should not have previously had any adverse reactions or resistance to bictegravir, emtricitabine, or tenofovir alafenamide.[citation needed]
Dosage
It comes in two different strengths.[1]
Combination therapy
Bictegravir/emtricitabine/tenofovir alafenamide is an example of a combination drug that can be taken as a complete regimen for treatment of the human immunodeficiency virus.[1]
Combination therapy for HIV, often called highly active antiretroviral therapy (HAART), is composed of two or more types of antiretroviral drugs. Combination therapy decreases the likelihood that drug resistance will occur, because it is unlikely that the HIV-1 strains will be able to mutate enough to become resistant to all drugs being used in the combination. Combination therapy increases the length of lives of patients with HIV-1, and can greatly reduce the possibility for transmission of the virus.[8]
Side effects
This drug should not be co-administered with dofetilide or rifampin. Dofetilide when taken with bictegravir/emtricitabine/tenofovir alafenamide can cause an increase in dofetilide plasma concentrations, which can lead to death. Rifampin and bictegravir/emtricitabine/tenofovir alafenamide when taken together can decrease bictegravir plasma concentrations and cause resistance to bictegravir/emtricitabine/tenofovir alafenamide. Other HIV-1 antiretroviral drugs should not be taken with this therapy.[citation needed]
If kidney disease or development of renal impairment is seen, the drug should be discontinued. Discontinuation of bictegravir/emtricitabine/tenofovir alafenamide in patients with hepatitis B and HIV-1 has been shown to increase the prevalence of hepatitis B, causing liver decompensation and liver failure[citation needed]
Adverse drug reactions include, but are not limited to, diarrhea, nausea, and headache.[1]
Components

- Bictegravir (BIC) is an integrase strand transfer inhibitor (INSTI). Bictegravir is different from other INSTIs because it contains a bridged bicyclic ring and a distinct benzyl tail with a 2,4,6-trifluorobenzyl group. This contributes to an increase in plasma protein binding and a reduction of activation of the pregnane X receptor (PXR). These changes minimize interactions between drugs, lower clearance, and increase solubility. Bictegravir was found to be less drug resistant than other drugs in the same class.[9]
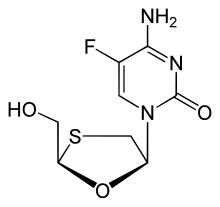
- Emtricitabine (FTC) is a nucleoside reverse transcriptase inhibitor (NRTI) that is a synthetic fluoro derivative of thiacytidine. Within the cell, emtricitabine becomes phosphorylated, which forms emitricitabine 5'-triphosphate within the cell. This allows for the drug to compete with the viral and host substrate and ultimately causes a termination of DNA chain elongation.[10] Underlying hepatitis B virus (HBV) can interact with emtricitabine to cause significant liver damage, but it does not have a significant detrimental effect on the liver when given to patients without HBV.[11]

- Tenofovir alafenamide (TAF) is a prodrug of tenofovir that functions as a nucleotide reverse transcriptase inhibitor (NRTI). Other prodrugs for tenofovir have been tested, but TAF is more efficient at refining HIV-1 therapy. It converts intracellularly to TFV diphosphate, which is a metabolite in HIV target cells.[12] Thus, TAF has higher active metabolite concentrations and lower plasma TFV than other Tenovir prodrugs.[13] TAF is metabolized primarily with the kidneys, and has a lower dosage than other prodrugs, so it is less detrimental to the renal elimination system.[12]
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 "Biktarvy- bictegravir sodium, emtricitabine, and tenofovir alafenamide fumarate tablet". DailyMed. 8 August 2019. Archived from the original on 1 August 2020. Retrieved 7 March 2020.
- ↑ 2.0 2.1 "Bictegravir / emtricitabine / tenofovir alafenamide (Biktarvy) Use During Pregnancy". Drugs.com. 16 September 2019. Archived from the original on 24 November 2020. Retrieved 7 March 2020.
- ↑ "Biktarvy 50 mg/200 mg/25 mg film-coated tablets - Summary of Product Characteristics (SmPC)". (emc). 25 August 2020. Archived from the original on 4 November 2021. Retrieved 26 August 2020.
- ↑ 4.0 4.1 BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 687. ISBN 978-0857114105.
- ↑ "Bictegravir / emtricitabine / tenofovir alafenamide (Biktarvy) Use During Pregnancy". Drugs.com. Archived from the original on 24 November 2020. Retrieved 10 January 2022.
- ↑ "Biktarvy EPAR". European Medicines Agency (EMA). 21 January 2020. Archived from the original on 11 August 2020. Retrieved 7 March 2020.
- ↑ "Biktarvy Prices, Coupons & Savings Tips - GoodRx". GoodRx. Archived from the original on 18 January 2024. Retrieved 10 January 2022.
- ↑ "FDA-Approved HIV Medicines Understanding HIV/AIDS". AIDSinfo. Archived from the original on 27 January 2021. Retrieved 22 May 2018.
- ↑ Tsiang M, Jones GS, Goldsmith J, Mulato A, Hansen D, Kan E, et al. (December 2016). "Antiviral Activity of Bictegravir (GS-9883), a Novel Potent HIV-1 Integrase Strand Transfer Inhibitor with an Improved Resistance Profile". Antimicrobial Agents and Chemotherapy. 60 (12): 7086–7097. doi:10.1128/AAC.01474-16. PMC 5118987. PMID 27645238.
- ↑ "Emtricitabine". Pubchem. U.S. National Library of Medicine. Archived from the original on 12 January 2021. Retrieved 22 May 2018.
- ↑ "Emtricitabine Dosage, Side Effects". AIDSinfo. Archived from the original on 1 September 2020. Retrieved 22 May 2018.
- ↑ 12.0 12.1 Ray AS, Fordyce MW, Hitchcock MJ (January 2016). "Tenofovir alafenamide: A novel prodrug of tenofovir for the treatment of Human Immunodeficiency Virus". Antiviral Research. 125: 63–70. doi:10.1016/j.antiviral.2015.11.009. PMID 26640223.
- ↑ "Tenofovir Alafenamide Information for Providers". AIDSinfo. Archived from the original on 2 September 2020. Retrieved 22 May 2018.
External links
| Identifiers: |
|---|
- "Bictegravir". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 24 May 2021. Retrieved 5 September 2021.
- "Emtricitabine". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 24 June 2021. Retrieved 5 September 2021.
- "Tenofovir alafenamide". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 24 June 2021. Retrieved 5 September 2021.
- Pages using duplicate arguments in template calls
- Use dmy dates from March 2020
- Articles with invalid date parameter in template
- Drugs with non-standard legal status
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Drugs missing an ATC code
- Drug has EMA link
- Articles containing unverified chemical infoboxes
- Drugs that are a combination of chemicals
- All articles with unsourced statements
- Articles with unsourced statements from May 2021
- Portal templates with all redlinked portals
- Fixed dose combination (antiretroviral)
- RTT