Osteoporosis
| Osteoporosis | |
|---|---|
 | |
| Elderly woman with osteoporosis showing a curved back from compression fractures of her back bones. | |
| Pronunciation | |
| Specialty | Rheumatology, orthopedics |
| Symptoms | Increased risk of a broken bone[3] |
| Complications | Chronic pain[3] |
| Usual onset | Older age[3] |
| Risk factors | Alcoholism, anorexia, hyperthyroidism, gastrointestinal diseases, surgical removal of the ovaries, kidney disease, smoking, certain medications[3] |
| Diagnostic method | Bone density scan[4] |
| Treatment | Good diet, exercise, fall prevention, stopping smoking[3] |
| Medication | Bisphosphonates[5][6] |
| Frequency | 15% (50 year olds), 70% (over 80 year olds)[7] |
Osteoporosis is a disease in which bone weakening increases the risk of a broken bone.[3] It is the most common reason for a broken bone among the elderly.[3] Bones that commonly break include the vertebrae in the spine, the bones of the forearm, and the hip.[8] Until a broken bone occurs there are typically no symptoms.[3] Bones may weaken to such a degree that a break may occur with minor stress or spontaneously.[3] After a broken bone, Chronic pain and a decreased ability to carry out normal activities may occur.[3]
Osteoporosis occurs when increased amount of bone is broken down by bone breaking cells and not enough made by bone making cells, resulting in loss of bone mass.[9] Bone loss increases after menopause due to lower levels of estrogen.[3] Osteoporosis may also occur due to a number of diseases or treatments, including alcoholism, anorexia, hyperthyroidism, kidney disease, and surgical removal of the ovaries.[3] Certain medications increase the rate of bone loss, including some antiseizure medications, chemotherapy, proton pump inhibitors, selective serotonin reuptake inhibitors, and glucocorticosteroids.[3] Smoking, and too little exercise are also risk factors.[3] Osteoporosis is defined as a bone density of 2.5 standard deviations below that of a young adult.[4] This is typically measured by dual-energy X-ray absorptiometry.[4]
Prevention of osteoporosis includes a proper diet during childhood and efforts to avoid medications that increase the rate of bone loss.[3] Efforts to prevent broken bones in those with osteoporosis include a good diet, exercise, and fall prevention.[3] Lifestyle changes such as stopping smoking and not drinking alcohol may help.[3] Biphosphonate medications are useful to decrease future broken bones in those with previous broken bones due to osteoporosis.[5][6] In those with osteoporosis but no previous broken bones, they are less effective.[5][6][10] They do not appear to affect the risk of death.[11] A number of other medications may also be useful.[3][12]
Osteoporosis becomes more common with age.[3] About 15% of Caucasians in their 50s and 70% of those over 80 are affected.[7] It is more common in women than men.[3] In the developed world, depending on the method of diagnosis, 2% to 8% of males and 9% to 38% of females are affected.[13] Rates of disease in the developing world are unclear.[14] About 22 million women and 5.5 million men in the European Union had osteoporosis in 2010.[15] In the United States in 2010, about eight million women and one to two million men had osteoporosis.[13][16] White and Asian people are at greater risk.[3] The word "osteoporosis" is from the Greek terms for "porous bones".[17]
Signs and symptoms

Osteoporosis itself has no symptoms; its main consequence is the increased risk of bone fractures. Osteoporotic fractures occur in situations where healthy people would not normally break a bone; they are therefore regarded as fragility fractures. Typical fragility fractures occur in the vertebral column, rib, hip and wrist.
Fractures
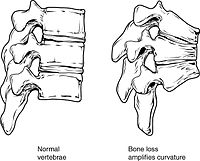
Fractures are a common symptom of osteoporosis and can result in disability.[18] Acute and chronic pain in the elderly is often attributed to fractures from osteoporosis and can lead to further disability and early mortality.[19] These fractures may also be asymptomatic. The most common osteoporotic fractures are of the wrist, spine, shoulder and hip. The symptoms of a vertebral collapse ("compression fracture") are sudden back pain, often with radicular pain (shooting pain due to nerve root compression) and rarely with spinal cord compression or cauda equina syndrome. Multiple vertebral fractures lead to a stooped posture, loss of height, and chronic pain with resultant reduction in mobility.[20]
Fractures of the long bones acutely impair mobility and may require surgery. Hip fracture, in particular, usually requires prompt surgery, as serious risks are associated with it, such as deep vein thrombosis and pulmonary embolism, and increased mortality.
Fracture risk calculators assess the risk of fracture based upon several criteria, including bone mineral density, age, smoking, alcohol usage, weight, and gender. Recognized calculators include FRAX[21] and Dubbo.
The term "established osteoporosis" is used when a broken bone due to osteoporosis has occurred.[22] Osteoporosis is a part of frailty syndrome.
Risk of falls

There is an increased risk of falls associated with aging. These falls can lead to skeletal damage at the wrist, spine, hip, knee, foot, and ankle. Part of the fall risk is because of impaired eyesight due to many causes, (e.g. glaucoma, macular degeneration), balance disorder, movement disorders (e.g. Parkinson's disease), dementia, and sarcopenia (age-related loss of skeletal muscle). Collapse (transient loss of postural tone with or without loss of consciousness). Causes of syncope are manifold, but may include cardiac arrhythmias (irregular heart beat), vasovagal syncope, orthostatic hypotension (abnormal drop in blood pressure on standing up), and seizures. Removal of obstacles and loose carpets in the living environment may substantially reduce falls. Those with previous falls, as well as those with gait or balance disorders, are most at risk.[23]
Risk factors
Risk factors for osteoporotic fracture can be split between nonmodifiable and (potentially) modifiable. In addition, osteoporosis is a recognized complication of specific diseases and disorders. Medication use is theoretically modifiable, although in many cases, the use of medication that increases osteoporosis risk may be unavoidable. Caffeine is not a risk factor for osteoporosis.[24]
It is more common in females than males.[3]
Nonmodifiable

- The most important risk factors for osteoporosis are advanced age (in both men and women) and female sex; estrogen deficiency following menopause or surgical removal of the ovaries is correlated with a rapid reduction in bone mineral density, while in men, a decrease in testosterone levels has a comparable (but less pronounced) effect.[26][27]
- Ethnicity: While osteoporosis occurs in people from all ethnic groups, European or Asian ancestry predisposes for osteoporosis.[28]
- Heredity: Those with a family history of fracture or osteoporosis are at an increased risk; the heritability of the fracture, as well as low bone mineral density, is relatively high, ranging from 25 to 80%. At least 30 genes are associated with the development of osteoporosis.[29]
- Those who have already had a fracture are at least twice as likely to have another fracture compared to someone of the same age and sex.[30]
- Build: A small stature is also a nonmodifiable risk factor associated with the development of osteoporosis.[31]
Potentially modifiable
- Excessive alcohol: Although small amounts of alcohol are probably beneficial (bone density increases with increasing alcohol intake), chronic heavy drinking (alcohol intake greater than three units/day) probably increases fracture risk despite any beneficial effects on bone density.[32][33]
- Vitamin D deficiency:[34][35] Low circulating Vitamin D is common among the elderly worldwide.[4] Mild vitamin D insufficiency is associated with increased parathyroid hormone (PTH) production.[4] PTH increases bone resorption, leading to bone loss. A positive association exists between serum 1,25-dihydroxycholecalciferol levels and bone mineral density, while PTH is negatively associated with bone mineral density.[4]
- Tobacco smoking: Many studies have associated smoking with decreased bone health, but the mechanisms are unclear. Tobacco smoking has been proposed to inhibit the activity of osteoblasts, and is an independent risk factor for osteoporosis.[32][36] Smoking also results in increased breakdown of exogenous estrogen, lower body weight and earlier menopause, all of which contribute to lower bone mineral density.[4]
- Malnutrition: Nutrition has an important and complex role in maintenance of good bone. Identified risk factors include low dietary calcium and/or phosphorus, magnesium, zinc, boron, iron, fluoride, copper, vitamins A, K, E and C (and D where skin exposure to sunlight provides an inadequate supply). Excess sodium is a risk factor. High blood acidity may be diet-related, and is a known antagonist of bone.[37] Some have identified low protein intake as associated with lower peak bone mass during adolescence and lower bone mineral density in elderly populations.[4] Conversely, some have identified low protein intake as a positive factor, protein is among the causes of dietary acidity. Imbalance of omega-6 to omega-3 polyunsaturated fats is yet another identified risk factor.[38]
- High dietary protein from animal sources: Research has found an association between diets high in animal protein and increased urinary calcium,[39][40][41] and have been linked to an increase in fractures.[42] A diet high in plant protein may be optimal for bone health, as higher protein diets tend to increase absorption of calcium from the diet and are associated with higher bone density.[43] Indeed, it has recently been argued that low protein diets cause poor bone health.[44] No interventional trials have been performed on dietary protein in the prevention and treatment of osteoporosis.[45]
- Underweight/inactive: Bone remodeling occurs in response to physical stress, so physical inactivity can lead to significant bone loss.[4] Weight bearing exercise can increase peak bone mass achieved in adolescence,[4] and a highly significant correlation between bone strength and muscle strength has been determined.[46] The incidence of osteoporosis is lower in overweight people.[47]
- Endurance training: In female endurance athletes, large volumes of training can lead to decreased bone density and an increased risk of osteoporosis.[48] This effect might be caused by intense training suppressing menstruation, producing amenorrhea, and it is part of the female athlete triad.[49] However, for male athletes, the situation is less clear, and although some studies have reported low bone density in elite male endurance athletes,[50] others have instead seen increased leg bone density.[51][52]
- Heavy metals: A strong association between cadmium and lead with bone disease has been established. Low-level exposure to cadmium is associated with an increased loss of bone mineral density readily in both genders, leading to pain and increased risk of fractures, especially in the elderly and in females. Higher cadmium exposure results in osteomalacia (softening of the bone).[53]
- Soft drinks: Some studies indicate soft drinks (many of which contain phosphoric acid) may increase risk of osteoporosis, at least in women.[54] Others suggest soft drinks may displace calcium-containing drinks from the diet rather than directly causing osteoporosis.[55]
- Proton pump inhibitors (such as lansoprazole, esomeprazole, or omeprazole) that decrease stomach acid, are a risk for bone fractures if taken for two or more years, due to decreased absorption of calcium in the stomach.[56]
Medical disorders

Many diseases and disorders have been associated with osteoporosis.[57] For some, the underlying mechanism influencing the bone metabolism is straightforward, whereas for others the causes are multiple or unknown.
- In general, immobilization causes bone loss (following the 'use it or lose it' rule). For example, localized osteoporosis can occur after prolonged immobilization of a fractured limb in a cast. This is also more common in active people with a high bone turn-over (for example, athletes). Other examples include bone loss during space flight or in people who are bedridden or use wheelchairs for various reasons.
- Hypogonadal states can cause secondary osteoporosis. These include Turner syndrome, Klinefelter syndrome, Kallmann syndrome, anorexia nervosa, andropause,[58] hypothalamic amenorrhea or hyperprolactinemia.[58] In females, the effect of hypogonadism is mediated by estrogen deficiency. It can appear as early menopause (<45 years) or from prolonged premenopausal amenorrhea (>1 year). Bilateral oophorectomy (surgical removal of the ovaries) and premature ovarian failure cause deficient estrogen production. In males, testosterone deficiency is the cause (for example, andropause or after surgical removal of the testes).
- Endocrine disorders that can induce bone loss include Cushing's syndrome,[4] hyperparathyroidism,[4] hyperthyroidism,[4] hypothyroidism, diabetes mellitus type 1 and 2,[59] acromegaly, and adrenal insufficiency.[57]
- Malnutrition, parenteral nutrition[4] and malabsorption can lead to osteoporosis. Nutritional and gastrointestinal disorders that can predispose to osteoporosis include undiagnosed and untreated coeliac disease (both symptomatic and asymptomatic people),[4][60] Crohn's disease,[61] ulcerative colitis,[61] cystic fibrosis,[61] surgery[58] (after gastrectomy, intestinal bypass surgery or bowel resection) and severe liver disease (especially primary biliary cirrhosis).[58] People with lactose intolerance or milk allergy may develop osteoporosis due to restrictions of calcium-containing foods.[62] Individuals with bulimia can also develop osteoporosis. Those with an otherwise adequate calcium intake can develop osteoporosis due to the inability to absorb calcium and/or vitamin D. Other micronutrients such as vitamin K or vitamin B12 deficiency may also contribute.
- People with rheumatologic disorders such as rheumatoid arthritis,[58] ankylosing spondylitis,[58] systemic lupus erythematosus and polyarticular juvenile idiopathic arthritis are at increased risk of osteoporosis, either as part of their disease or because of other risk factors (notably corticosteroid therapy). Systemic diseases such as amyloidosis and sarcoidosis can also lead to osteoporosis.
- Chronic kidney disease can lead to renal osteodystrophy.
- Hematologic disorders linked to osteoporosis are multiple myeloma[58] and other monoclonal gammopathies,[59] lymphoma, leukemia, mastocytosis,[58] hemophilia, sickle-cell disease and thalassemia.
- Several inherited disorders have been linked to osteoporosis. These include osteogenesis imperfecta,[58] Marfan syndrome,[58] hemochromatosis,[4] hypophosphatasia[63] (for which it is often misdiagnosed),[64] glycogen storage diseases, homocystinuria,[58] Ehlers–Danlos syndrome,[58] porphyria, Menkes' syndrome, epidermolysis bullosa and Gaucher's disease.
- People with scoliosis of unknown cause also have a higher risk of osteoporosis. Bone loss can be a feature of complex regional pain syndrome. It is also more frequent in people with Parkinson's disease and chronic obstructive pulmonary disease.
- People with Parkinson's disease have a higher risk of broken bones. This is related to poor balance and poor bone density.[65] In Parkinson's disease there may be a link between the loss of dopaminergic neurons and altered calcium metabolism[66] (and iron metabolism) causing a stiffening of the skeleton and kyphosis.
Medication
Certain medications have been associated with an increase in osteoporosis risk; only glucocorticosteroids and anticonvulsants are classically associated, but evidence is emerging with regard to other drugs.
- Steroid-induced osteoporosis (SIOP) arises due to use of glucocorticoids – analogous to Cushing's syndrome and involving mainly the axial skeleton. The synthetic glucocorticoid prescription drug prednisone is a main candidate after prolonged intake. Some professional guidelines recommend prophylaxis in patients who take the equivalent of more than 30 mg hydrocortisone (7.5 mg of prednisolone), especially when this is in excess of three months.[67] It is recommended to use calcium or Vitamin D as prevention.[68] Alternate day use may not prevent this complication.[69]
- Barbiturates, phenytoin and some other enzyme-inducing antiepileptics – these probably accelerate the metabolism of vitamin D.[70]
- L-Thyroxine over-replacement may contribute to osteoporosis, in a similar fashion as thyrotoxicosis does.[57] This can be relevant in subclinical hypothyroidism.
- Several drugs induce hypogonadism, for example aromatase inhibitors used in breast cancer, methotrexate and other antimetabolite drugs, depot progesterone and gonadotropin-releasing hormone agonists.
- Anticoagulants – long-term use of heparin is associated with a decrease in bone density,[71] and warfarin (and related coumarins) have been linked with an increased risk in osteoporotic fracture in long-term use.[72]
- Proton pump inhibitors – these drugs inhibit the production of stomach acid; this is thought to interfere with calcium absorption.[73] Chronic phosphate binding may also occur with aluminium-containing antacids.[57]
- Thiazolidinediones (used for diabetes) – rosiglitazone and possibly pioglitazone, inhibitors of PPARγ, have been linked with an increased risk of osteoporosis and fracture.[74]
- Chronic lithium therapy has been associated with osteoporosis.[57]
Evolutionary
Age-related bone loss is common among humans due to exhibiting less dense bones than other primate species.[75] Because of the more porous bones of humans, frequency of severe osteoporosis and osteoporosis related fractures is higher.[76] The human vulnerability to osteoporosis is an obvious cost but it can be justified by the advantage of bipedalism inferring that this vulnerability is the byproduct of such.[76] It has been suggested that porous bones help to absorb the increased stress that we have on two surfaces compared to our primate counterparts who have four surfaces to disperse the force.[75] In addition, the porosity allows for more flexibility and a lighter skeleton that is easier to support.[76] One other consideration may be that diets today have much lower amounts of calcium than the diets of other primates or the tetrapedal ancestors to humans which may lead to higher likelihood to show signs of osteoporosis.[77]
Pathogenesis

The underlying mechanism in all cases of osteoporosis is an imbalance between bone resorption and bone formation. In normal bone, matrix remodeling of bone is constant; up to 10% of all bone mass may be undergoing remodeling at any point in time. The process takes place in bone multicellular units (BMUs) as first described by Frost & Thomas in 1963.[78] Osteoclasts are assisted by transcription factor PU.1 to degrade the bone matrix, while osteoblasts rebuild the bone matrix. Low bone mass density can then occur when osteoclasts are degrading the bone matrix faster than the osteoblasts are rebuilding the bone.[79]
The three main mechanisms by which osteoporosis develops are an inadequate peak bone mass (the skeleton develops insufficient mass and strength during growth), excessive bone resorption, and inadequate formation of new bone during remodeling, likely due to mesenchymal stem cells biasing away from the osteoblast and toward the marrow adipocyte lineage.[80] An interplay of these three mechanisms underlies the development of fragile bone tissue.[29] Hormonal factors strongly determine the rate of bone resorption; lack of estrogen (e.g. as a result of menopause) increases bone resorption, as well as decreasing the deposition of new bone that normally takes place in weight-bearing bones. The amount of estrogen needed to suppress this process is lower than that normally needed to stimulate the uterus and breast gland. The α-form of the estrogen receptor appears to be the most important in regulating bone turnover.[29] In addition to estrogen, calcium metabolism plays a significant role in bone turnover, and deficiency of calcium and vitamin D leads to impaired bone deposition; in addition, the parathyroid glands react to low calcium levels by secreting parathyroid hormone (parathormone, PTH), which increases bone resorption to ensure sufficient calcium in the blood. The role of calcitonin, a hormone generated by the thyroid that increases bone deposition, is less clear and probably not as significant as that of PTH.[29]
The activation of osteoclasts is regulated by various molecular signals, of which RANKL (receptor activator of nuclear factor kappa-B ligand) is one of the best studied. This molecule is produced by osteoblasts and other cells (e.g. lymphocytes), and stimulates RANK (receptor activator of nuclear factor κB). Osteoprotegerin (OPG) binds RANKL before it has an opportunity to bind to RANK, and hence suppresses its ability to increase bone resorption. RANKL, RANK and OPG are closely related to tumor necrosis factor and its receptors. The role of the Wnt signaling pathway is recognized, but less well understood. Local production of eicosanoids and interleukins is thought to participate in the regulation of bone turnover, and excess or reduced production of these mediators may underlie the development of osteoporosis.[29]
Trabecular bone (or cancellous bone) is the sponge-like bone in the ends of long bones and vertebrae. Cortical bone is the hard outer shell of bones and the middle of long bones. Because osteoblasts and osteoclasts inhabit the surface of bones, trabecular bone is more active and is more subject to bone turnover and remodeling. Not only is bone density decreased, but the microarchitecture of bone is also disrupted. The weaker spicules of trabecular bone break ("microcracks"), and are replaced by weaker bone. Common osteoporotic fracture sites, the wrist, the hip and the spine, have a relatively high trabecular bone to cortical bone ratio. These areas rely on the trabecular bone for strength, so the intense remodeling causes these areas to degenerate most when the remodeling is imbalanced.[citation needed] Around the ages of 30–35, cancellous or trabecular bone loss begins. Women may lose as much as 50%, while men lose about 30%.[31]
-
Light micrograph of an osteoclast displaying typical distinguishing characteristics: a large cell with multiple nuclei and a "foamy" cytosol.
-
Light micrograph of osteoblasts, several displaying a prominent Golgi apparatus, actively synthesizing osteoid containing two osteocytes.
-
Collapse of vertebra on the right, normal on the left
Diagnosis
The diagnosis of osteoporosis can be made using conventional radiography and by measuring the bone mineral density (BMD).[81] The most popular method of measuring BMD is dual-energy X-ray absorptiometry.
In addition to the detection of abnormal BMD, the diagnosis of osteoporosis requires investigations into potentially modifiable underlying causes; this may be done with blood tests. Depending on the likelihood of an underlying problem, investigations for cancer with metastasis to the bone, multiple myeloma, Cushing's disease and other above-mentioned causes may be performed.
Conventional radiography
-
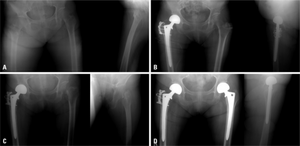
a) Initial radiographs of intertrochanteric fracture b) postoperative - bipolar hemiarthroplasty c) subsequent fracture in left femur neck d) radiograph after a bipolar hemiarthroplasty, the individual began medication for osteoporosis
-
Multiple osteoporotic wedge fractures demonstrated on a lateral thoraco-lumbar spine X-ray
Conventional radiography is useful, both by itself and in conjunction with CT or MRI, for detecting complications of osteopenia (reduced bone mass; pre-osteoporosis), such as fractures; for differential diagnosis of osteopenia; or for follow-up examinations in specific clinical settings, such as soft tissue calcifications, secondary hyperparathyroidism, or osteomalacia in renal osteodystrophy. However, radiography is relatively insensitive to detection of early disease and requires a substantial amount of bone loss (about 30%) to be apparent on X-ray images.
The main radiographic features of generalized osteoporosis are cortical thinning and increased radiolucency. Frequent complications of osteoporosis are vertebral fractures for which spinal radiography can help considerably in diagnosis and follow-up. Vertebral height measurements can objectively be made using plain-film X-rays by using several methods such as height loss together with area reduction, particularly when looking at vertical deformity in T4-L4, or by determining a spinal fracture index that takes into account the number of vertebrae involved. Involvement of multiple vertebral bodies leads to kyphosis of the thoracic spine, leading to what is known as dowager's hump.
Dual-energy X-ray
Dual-energy X-ray absorptiometry (DEXA scan) is considered the gold standard for the diagnosis of osteoporosis. Osteoporosis is diagnosed when the bone mineral density is less than or equal to 2.5 standard deviations below that of a young (30–40-year-old[4]:58), healthy adult women reference population. This is translated as a T-score. But because bone density decreases with age, more people become osteoporotic with increasing age.[4]:58 The World Health Organization has established the following diagnostic guidelines:[4][22]
| Category | T-score range | % young women |
|---|---|---|
| Normal | T-score ≥ −1.0 | 85% |
| Osteopenia | −2.5 < T-score < −1.0 | 14% |
| Osteoporosis | T-score ≤ −2.5 | 0.6% |
| Severe osteoporosis | T-score ≤ −2.5 with fragility fracture[22] |
The International Society for Clinical Densitometry takes the position that a diagnosis of osteoporosis in men under 50 years of age should not be made on the basis of densitometric criteria alone. It also states, for premenopausal women, Z-scores (comparison with age group rather than peak bone mass) rather than T-scores should be used, and the diagnosis of osteoporosis in such women also should not be made on the basis of densitometric criteria alone.[82]
Biomarkers
Chemical biomarkers are a useful tool in detecting bone degradation. The enzyme cathepsin K breaks down type-I collagen, an important constituent in bones. Prepared antibodies can recognize the resulting fragment, called a neoepitope, as a way to diagnose osteoporosis.[83] Increased urinary excretion of C-telopeptides, a type-I collagen breakdown product, also serves as a biomarker for osteoporosis.[84]
| Condition | Calcium | Phosphate | Alkaline phosphatase | Parathyroid hormone | Comments |
|---|---|---|---|---|---|
| Osteopenia | unaffected | unaffected | normal | unaffected | decreased bone mass |
| Osteopetrosis | unaffected | unaffected | elevated | unaffected[citation needed] | thick dense bones also known as marble bone |
| Osteomalacia and rickets | decreased | decreased | elevated | elevated | soft bones |
| Osteitis fibrosa cystica | elevated | decreased | elevated | elevated | brown tumors |
| Paget's disease of bone | unaffected | unaffected | variable (depending on stage of disease) | unaffected | abnormal bone architecture |
Other measuring tools
Quantitative computed tomography (QCT) differs from DXA in that it gives separate estimates of BMD for trabecular and cortical bone and reports precise volumetric mineral density in mg/cm3 rather than BMD's relative Z-score. Among QCT's advantages: it can be performed at axial and peripheral sites, can be calculated from existing CT scans without a separate radiation dose, is sensitive to change over time, can analyze a region of any size or shape, excludes irrelevant tissue such as fat, muscle, and air, and does not require knowledge of the patient's subpopulation in order to create a clinical score (e.g. the Z-score of all females of a certain age). Among QCT's disadvantages: it requires a high radiation dose compared to DXA, CT scanners are large and expensive, and because its practice has been less standardized than BMD, its results are more operator-dependent. Peripheral QCT has been introduced to improve upon the limitations of DXA and QCT.[81]
Quantitative ultrasound has many advantages in assessing osteoporosis. The modality is small, no ionizing radiation is involved, measurements can be made quickly and easily, and the cost of the device is low compared with DXA and QCT devices. The calcaneus is the most common skeletal site for quantitative ultrasound assessment because it has a high percentage of trabecular bone that is replaced more often than cortical bone, providing early evidence of metabolic change. Also, the calcaneus is fairly flat and parallel, reducing repositioning errors. The method can be applied to children, neonates, and preterm infants, just as well as to adults.[81] Some ultrasound devices can be used on the tibia.[85]
Screening
The U.S. Preventive Services Task Force (USPSTF) recommend that all women 65 years of age or older be screened by bone densitometry.[86] Additionally they recommend screening younger women with risk factors.[86] There is insufficient evidence to make recommendations about the intervals for repeated screening and the appropriate age to stop screening.[87]
In men the harm versus benefit of screening for osteoporosis is unknown.[86] Prescrire states that the need to test for osteoporosis in those who have not had a previous bone fracture is unclear.[88] The International Society for Clinical Densitometry suggest BMD testing for men 70 or older, or those who are indicated for risk equal to that of a 70‑year‑old.[89] A number of tools exist to help determine who is reasonable to test.[90]
Prevention
Lifestyle prevention of osteoporosis is in many aspects the inverse of the potentially modifiable risk factors.[91] As tobacco smoking and high alcohol intake have been linked with osteoporosis, smoking cessation and moderation of alcohol intake are commonly recommended as ways to help prevent it.[92]
In people with coeliac disease adherence to a gluten-free diet decreases the risk of developing osteoporosis[93] and increases bone density.[60] The diet must ensure optimal calcium intake (of at least one gram daily) and measuring vitamin D levels is recommended, and to take specific supplements if necessary.[93]
Nutrition
Studies of the benefits of supplementation with calcium and vitamin D are conflicting, possibly because most studies did not have people with low dietary intakes.[97] A 2018 review by the USPSTF found low-quality evidence that the routine use of calcium and vitamin D supplements (or both supplements together) did not reduce the risk of having an osteoporotic fracture in male and female adults living in the community who had no known history of vitamin D deficiency, osteoporosis, or a fracture.[98] Furthermore, the same review found moderate-quality evidence that the combination of vitamin D and calcium supplementation increases the risk for developing kidney stones in this population.[98] The evidence was insufficient to determine if supplementation with vitamin D, calcium, or the combination of both had an effect on the risk of cancer, cardiovascular disease, or death from any cause.[98] The USPSTF does not recommend low dose supplementation (less than 1 g of calcium and 400 IU of vitamin D) in postmenopausal women as there does not appear to be a difference in fracture risk.[99] A 2015 review found little data that supplementation of calcium decreases the risk of fractures.[100]
While some meta-analyses have found a benefit of vitamin D supplements combined with calcium for fractures, they did not find a benefit of vitamin D supplements (800 IU/day or less) alone.[101][102]
While supplementation does not appear to affect the risk of death,[98][102] there is an increased risk of myocardial infarctions with calcium supplementation,[103][104] kidney stones,[98] and stomach problems.[102]
Vitamin K deficiency is also a risk factor for osteoporotic fractures. The gene gamma-glutamyl carboxylase (GGCX) is dependent on vitamin K. Functional polymorphisms in the gene could attribute to variation in bone metabolism and BMD. Vitamin K2 is also used as a means of treatment for osteoporosis and the polymorphisms of GGCX could explain the individual variation in the response to treatment of vitamin K.[105]
Good dietary sources of calcium include dairy leafy greens, legumes, and beans.[106] There is conflicting evidence about whether or not dairy is an adequate source of calcium to prevent fractures. The National Academy of Sciences recommends 1,000 mg of calcium for those ages 19–50, and 1,200 mg for those ages 50 and above. However, this would equate to 2-3 glasses of milk, which is over the required amount or a healthy diet. Currently, there is not sufficient evidence to show that drinking more than 1 glass of milk a day prevents fractures, and due to evidence about possible increased risks of ovarian and prostate cancer with increased dairy, it is recommended to avoid high intakes of dairy.[107]
Physical exercise
There is limited evidence indicating that exercise is helpful in promoting bone health.[108] A 2011 review reported a small benefit of physical exercise on bone density of postmenopausal women.[109] The chances of having a fracture were also slightly reduced (absolute difference 4%).[109] People who exercised had on average less bone loss (0.85% at the spine, 1.03% at the hip).[109] However, other studies suggest that increased bone activity and weight-bearing exercises at a young age prevent bone fragility in adults.[110]
Low-quality evidence suggests that exercise may improve pain and quality of life of people with vertebral fractures.[111] Moderate-quality evidence found that exercise will likely improve physical performance in individuals with vertebral fractures.[112]
Physical therapy
People with osteoporosis are at higher risk of falls due to poor postural control, muscle weakness, and overall deconditioning.[113] Postural control is important to maintaining functional movements such as walking and standing. Physical therapy may be an effective way to address postural weakness that may result from vertebral fractures, which are common in people with osteoporosis. Physical therapy treatment plans for people with vertebral fractures include balance training, postural correction, trunk and lower extremity muscle strengthening exercises, and moderate-intensity aerobic physical activity.[114]. The goal of these interventions are to regain normal spine curvatures, increase spine stability, and improve functional performance.[115] Physical therapy interventions were also designed to slow the rate of bone loss through home exercise programs.[116]
Management
Lifestyle
Weight-bearing endurance exercise and/or exercises to strengthen muscles improve bone strength in those with osteoporosis.[109][117] Aerobics, weight bearing, and resistance exercises all maintain or increase BMD in postmenopausal women.[109] Fall prevention can help prevent osteoporosis complications. There is some evidence for hip protectors specifically among those who are in care homes.[118]
Medications
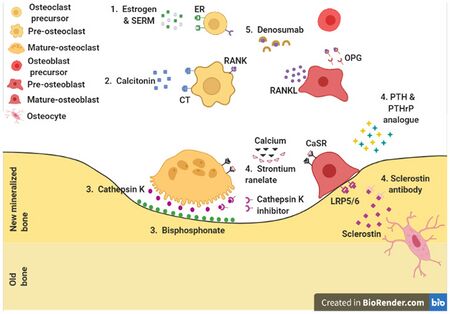
Bisphosphonates are useful in decreasing the risk of future fractures in those who have already sustained a fracture due to osteoporosis.[5][6][92] This benefit is present when taken for three to four years.[120][121] They do not appear to change the overall risk of death.[122] Tentative evidence does not support the use of bisphosphonates as a standard treatment for secondary osteoporosis in children.[121] Different bisphosphonates have not been directly compared, therefore it is unknown if one is better than another.[92] Fracture risk reduction is between 25 and 70% depending on the bone involved.[92] There are concerns of atypical femoral fractures and osteonecrosis of the jaw with long-term use, but these risks are low.[92][123] With evidence of little benefit when used for more than three to five years and in light of the potential adverse events, it may be appropriate to stop treatment after this time.[120] One medical organization recommends that after five years of medications by mouth or three years of intravenous medication among those at low risk, bisphosphonate treatment can be stopped.[124][125] In those at higher risk they recommend up to ten years of medication by mouth or six years of intravenous treatment.[124]
For those with osteoporosis but who have not had a fracture, evidence does not support a reduction in fracture risk with risedronate[6] or etidronate.[10][126] Alendronate decreases fractures of the spine but does not have any effect on other types of fractures.[5] Half stop their medications within a year.[127] When on treatment with bisphosphonates rechecking bone mineral density is not needed.[125] Another review found tentative evidence of benefit in males with osteoporosis.[128]
Fluoride supplementation does not appear to be effective in postmenopausal osteoporosis, as even though it increases bone density, it does not decrease the risk of fractures.[129][130]
Teriparatide (a recombinant parathyroid hormone) has been shown to be effective in treatment of women with postmenopausal osteoporosis.[131] Some evidence also indicates strontium ranelate is effective in decreasing the risk of vertebral and nonvertebral fractures in postmenopausal women with osteoporosis.[132] Hormone replacement therapy, while effective for osteoporosis, is only recommended in women who also have menopausal symptoms.[92] It is not recommended for osteoporosis by itself.[125] Raloxifene, while effective in decreasing vertebral fractures, does not affect the risk of nonvertebral fracture.[92] And while it reduces the risk of breast cancer, it increases the risk of blood clots and strokes.[92] While denosumab is effective at preventing fractures in women,[92] there is not clear evidence of benefit in males.[128] In hypogonadal men, testosterone has been shown to improve bone quantity and quality, but, as of 2008, no studies evaluated its effect on fracture risk or in men with a normal testosterone levels.[59] Calcitonin while once recommended is no longer due to the associated risk of cancer and questionable effect on fracture risk.[133] Alendronic acid/colecalciferol can be taken to treat this condition in post-menopausal women[citation needed]
Certain medications like alendronate, etidronate, risedronate, raloxifene, and strontium ranelate can help to prevent osteoporotic fragility fractures in postmenopausal women with osteoporosis.[134] Tentative evidence suggests that Chinese herbal medicines may have potential benefits on bone mineral density.[135]
Prognosis
| WHO category | Age 50–64 | Age > 64 | Overall |
|---|---|---|---|
| Normal | 5.3 | 9.4 | 6.6 |
| Osteopenia | 11.4 | 19.6 | 15.7 |
| Osteoporosis | 22.4 | 46.6 | 40.6 |
Although people with osteoporosis have increased mortality due to the complications of fracture, the fracture itself is rarely lethal.
Hip fractures can lead to decreased mobility and additional risks of numerous complications (such as deep venous thrombosis and/or pulmonary embolism, and pneumonia). The six-month mortality rate for those aged 50 and above following hip fracture was found to be around 13.5%, with a substantial proportion (almost 13%) needing total assistance to mobilize after a hip fracture.[137]
Vertebral fractures, while having a smaller impact on mortality, can lead to a severe chronic pain of neurogenic origin, which can be hard to control, as well as deformity. Though rare, multiple vertebral fractures can lead to such severe hunch back (kyphosis), the resulting pressure on internal organs can impair one's ability to breathe.
Apart from risk of death and other complications, osteoporotic fractures are associated with a reduced health-related quality of life.[138]
The condition is responsible for millions of fractures annually, mostly involving the lumbar vertebrae, hip, and wrist. Fragility fractures of ribs are also common in men.
Hip fractures
Hip fractures are responsible for the most serious consequences of osteoporosis. In the United States, more than 250,000 hip fractures annually are attributable to osteoporosis.[139] A 50-year-old white woman is estimated to have a 17.5% lifetime risk of fracture of the proximal femur. The incidence of hip fractures increases each decade from the sixth through the ninth for both women and men for all populations. The highest incidence is found among men and women ages 80 or older.[140]
Vertebral fractures
Between 35 and 50% of all women over 50 had at least one vertebral fracture. In the United States, 700,000 vertebral fractures occur annually, but only about a third are recognized. In a series of 9704 women aged 68.8 on average studied for 15 years, 324 had already suffered a vertebral fracture at entry into the study and 18.2% developed a vertebral fracture, but that risk rose to 41.4% in women who had a previous vertebral fracture.[141]
Wrist fractures
In the United States, 250,000 wrist fractures annually are attributable to osteoporosis.[139] Wrist fractures are the third most common type of osteoporotic fractures. The lifetime risk of sustaining a Colles' fracture is about 16% for white women. By the time women reach age 70, about 20% have had at least one wrist fracture.[140]
Rib fractures
Fragility fractures of the ribs are common in men as young as age 35. These are often overlooked as signs of osteoporosis, as these men are often physically active and suffer the fracture in the course of physical activity. An example would be as a result of falling while water skiing or jet skiing. However, a quick test of the individual's testosterone level following the diagnosis of the fracture will readily reveal whether that individual might be at risk.
Epidemiology

Globally it is estimated that 200 million people have osteoporosis.[143] Osteoporosis becomes more common with age.[3] About 15% of Caucasians in their 50s and 70% of those over 80 are affected.[7] It is more common in women than men.[3] In the developed world, depending on the method of diagnosis, 2% to 8% of males and 9% to 38% of females are affected.[13] Rates of disease in the developing world are unclear.[14]
Postmenopausal women have a higher rate of osteoporosis and fractures than older men.[144] Postmenopausal women have decreased estrogen which contributes to their higher rates of osteoporosis.[144] A 60-year-old women has a 44% risk of fracture while a 60-year-old man has a 25% risk of fracture.[144]
There are 8.9 million fractures worldwide per year due to osteoporosis.[145] Globally, 1 in 3 women and 1 in 5 men over the age of 50 will have an osteoporotic fracture.[145] Data from the United States shows a decrease in osteoporosis within the general population and in white women, from 18% in 1994 to 10% in 2006.[146] White and Asian people are at greater risk.[3] People of African descent are at a decreased risk of fractures due to osteoporosis, although they have the highest risk of death following an osteoporotic fracture.[146]
It has been shown that latitude affects risk of osteoporotic fracture.[142] Areas of higher latitude such as Northern Europe receive less Vitamin D through sunlight compared to regions closer to the equator, and consequently have higher fracture rates in comparison to lower latitudes.[142] For example, Swedish men and women have a 13% and 28.5% risk of hip fracture by age 50, respectively, whereas this risk is only 1.9% and 2.4% in Chinese men and women.[146] Diet may also be a factor that is responsible for this difference, as vitamin D, calcium, magnesium, and folate are all linked to bone mineral density.[147]
There is also an association between Celiac Disease and increased risk of osteoporosis.[148] In studies with premenopausal females and males, there was a correlation between Celiac Disease and osteoporosis and osteopenia.[149] Celiac Disease can decrease absorption of nutrients in the small intestine such as calcium, and a gluten-free diet can help people with Celiac Disease to revert to normal absorption in the gut.[150]
About 22 million women and 5.5 million men in the European Union had osteoporosis in 2010.[15] In the United States in 2010 about 8 million women and one to 2 million men had osteoporosis.[13][16] This places a large economic burden on the healthcare system due to costs of treatment, long-term disability, and loss of productivity in the working population. The EU spends 37 billion euros per year in healthcare costs related to osteoporosis, and the US spends an estimated US$19 billion annually for related healthcare costs.[145]
History
The link between age-related reductions in bone density and fracture risk goes back at least to Astley Cooper, and the term "osteoporosis" and recognition of its pathological appearance is generally attributed to the French pathologist Jean Lobstein.[151] The American endocrinologist Fuller Albright linked osteoporosis with the postmenopausal state.[152] Bisphosphonates were discovered in the 1960s.[153]
Anthropologists have studied skeletal remains that showed loss of bone density and associated structural changes that were linked to a chronic malnutrition in the agricultural area in which these individuals lived. "It follows that the skeletal deformation may be attributed to their heavy labor in agriculture as well as to their chronic malnutrition", causing the osteoporosis seen when radiographs of the remains were made.[154]
Osteoporosis means "porous bones", from Greek: οστούν/ostoun meaning "bone" and πόρος/poros meaning "pore".
References
- ↑ Jones, Daniel (2003) [1917], Roach, Peter; Hartmann, James; Setter, Jane (eds.), English Pronouncing Dictionary, Cambridge: Cambridge University Press, ISBN 978-3-12-539683-8
- ↑ "Osteoporosis". Merriam-Webster Dictionary.
- ↑ 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 3.11 3.12 3.13 3.14 3.15 3.16 3.17 3.18 3.19 3.20 3.21 3.22 3.23 3.24 "Handout on Health: Osteoporosis". NIAMS. August 2014. Archived from the original on 18 May 2015. Retrieved 16 May 2015.
- ↑ 4.00 4.01 4.02 4.03 4.04 4.05 4.06 4.07 4.08 4.09 4.10 4.11 4.12 4.13 4.14 4.15 4.16 4.17 4.18 WHO Scientific Group on the Prevention and Management of Osteoporosis (2000 : Geneva, Switzerland) (2003). Prevention and management of osteoporosis : report of a WHO scientific group (PDF). pp. 7, 31. ISBN 978-9241209212. Archived (PDF) from the original on 16 July 2007.
- ↑ 5.0 5.1 5.2 5.3 5.4 Wells GA, Cranney A, Peterson J, Boucher M, Shea B, Robinson V, Coyle D, Tugwell P (January 2008). "Alendronate for the primary and secondary prevention of osteoporotic fractures in postmenopausal women". The Cochrane Database of Systematic Reviews (1): CD001155. doi:10.1002/14651858.CD001155.pub2. PMID 18253985.
- ↑ 6.0 6.1 6.2 6.3 6.4 Wells G, Cranney A, Peterson J, Boucher M, Shea B, Robinson V, Coyle D, Tugwell P (January 2008). "Risedronate for the primary and secondary prevention of osteoporotic fractures in postmenopausal women". The Cochrane Database of Systematic Reviews (1): CD004523. doi:10.1002/14651858.CD004523.pub3. PMID 18254053.
- ↑ 7.0 7.1 7.2 "Chronic rheumatic conditions". World Health Organization. Archived from the original on 27 April 2015. Retrieved 18 May 2015.
- ↑ Golob AL, Laya MB (May 2015). "Osteoporosis: screening, prevention, and management". The Medical Clinics of North America. 99 (3): 587–606. doi:10.1016/j.mcna.2015.01.010. PMID 25841602. Archived from the original on 6 August 2020. Retrieved 2 August 2020.
- ↑ Conway, Richard (2020). "19. Bone disease". In Feather, Adam; Randall, David; Waterhouse, Mona (eds.). Kumar and Clark's Clinical Medicine (10th ed.). Elsevier. pp. 477–482. ISBN 978-0-7020-7870-5. Archived from the original on 12 December 2021. Retrieved 12 December 2021.
- ↑ 10.0 10.1 Wells GA, Cranney A, Peterson J, Boucher M, Shea B, Robinson V, Coyle D, Tugwell P (January 2008). "Etidronate for the primary and secondary prevention of osteoporotic fractures in postmenopausal women". The Cochrane Database of Systematic Reviews (1): CD003376. doi:10.1002/14651858.CD003376.pub3. PMC 6999803. PMID 18254018.
- ↑ Cummings, SR; Lui, LY; Eastell, R; Allen, IE (19 August 2019). "Association Between Drug Treatments for Patients With Osteoporosis and Overall Mortality Rates: A Meta-analysis". JAMA Internal Medicine. doi:10.1001/jamainternmed.2019.2779. PMC 6704731. PMID 31424486.
- ↑ Nelson HD, Haney EM, Chou R, Dana T, Fu R, Bougatsos C (2010). "Screening for Osteoporosis: Systematic Review to Update the 2002 U.S. Preventive Services Task Force Recommendation [Internet]". Agency for Healthcare Research and Quality. PMID 20722176.
- ↑ 13.0 13.1 13.2 13.3 Wade SW, Strader C, Fitzpatrick LA, Anthony MS, O'Malley CD (2014). "Estimating prevalence of osteoporosis: examples from industrialized countries". Archives of Osteoporosis. 9 (1): 182. doi:10.1007/s11657-014-0182-3. PMID 24847682. S2CID 19534928.
- ↑ 14.0 14.1 Handa R, Ali Kalla A, Maalouf G (August 2008). "Osteoporosis in developing countries". Best Practice & Research. Clinical Rheumatology. 22 (4): 693–708. doi:10.1016/j.berh.2008.04.002. PMID 18783745.
- ↑ 15.0 15.1 Svedbom A, Hernlund E, Ivergård M, Compston J, Cooper C, Stenmark J, McCloskey EV, Jönsson B, Kanis JA (2013). "Osteoporosis in the European Union: a compendium of country-specific reports". Archives of Osteoporosis. 8 (1–2): 137. doi:10.1007/s11657-013-0137-0. PMC 3880492. PMID 24113838.
- ↑ 16.0 16.1 Willson T, Nelson SD, Newbold J, Nelson RE, LaFleur J (2015). "The clinical epidemiology of male osteoporosis: a review of the recent literature". Clinical Epidemiology. 7: 65–76. doi:10.2147/CLEP.S40966. PMC 4295898. PMID 25657593.
- ↑ King, Tekoa L.; Brucker, Mary C. (2011). Pharmacology for women's health. Sudbury, Mass.: Jones and Bartlett Publishers. p. 1004. ISBN 9780763753290. Archived from the original on 8 September 2017.
- ↑ Jameson, J Larry; Kasper, Dennis L; Longo, Dan L; Fauci, Anthony S; Hauser, Stephen L; Loscalzo, Joseph; Harrison, Tinsley Randolph (6 February 2018). Harrison's principles of internal medicine (Twentieth ed.). New York. ISBN 9781259644047. OCLC 990065894.
- ↑ Old JL, Calvert M (2004). "Vertebral compression fractures in the elderly". American Family Physician. 69 (1): 111–16. PMID 14727827. Archived from the original on 5 August 2011. Retrieved 31 March 2011.
- ↑ Kim DH, Vaccaro AR (2006). "Osteoporotic compression fractures of the spine; current options and considerations for treatment". The Spine Journal. 6 (5): 479–87. doi:10.1016/j.spinee.2006.04.013. PMID 16934715.
- ↑ Susan Ott. "Fracture Risk Calculator". Archived from the original on 14 October 2009. Retrieved 3 November 2009.
- ↑ 22.0 22.1 22.2 WHO (1994). "Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group". World Health Organization Technical Report Series. 843: 1–129. PMID 7941614.
- ↑ Ganz DA, Bao Y, Shekelle PG, Rubenstein LZ (2007). "Will my patient fall?". JAMA. 297 (1): 77–86. doi:10.1001/jama.297.1.77. PMID 17200478.
- ↑ Waugh EJ, Lam MA, Hawker GA, McGowan J, Papaioannou A, Cheung AM, Hodsman AB, Leslie WD, Siminoski K, Jamal SA (January 2009). "Risk factors for low bone mass in healthy 40–60 year old women: a systematic review of the literature". Osteoporosis International. 20 (1): 1–21. doi:10.1007/s00198-008-0643-x. PMC 5110317. PMID 18523710.
- ↑ "6.6 Exercise, Nutrition, Hormones, and Bone Tissue". Anatomy & Physiology. Openstax CNX. 2013. ISBN 978-1-938168-13-0. Archived from the original on 10 January 2017.
- ↑ Sinnesael M, Claessens F, Boonen S, Vanderschueren D (2013). "Novel insights in the regulation and mechanism of androgen action on bone". Current Opinion in Endocrinology, Diabetes and Obesity. 20 (3): 240–44. doi:10.1097/MED.0b013e32835f7d04. PMID 23449008. S2CID 1637184.
- ↑ Sinnesael M, Boonen S, Claessens F, Gielen E, Vanderschueren D (2011). "Testosterone and the male skeleton: a dual mode of action". Journal of Osteoporosis. 2011: 1–7. doi:10.4061/2011/240328. PMC 3173882. PMID 21941679.
- ↑ Melton LJ (2003). "Epidemiology worldwide". Endocrinol. Metab. Clin. North Am. 32 (1): v, 1–13. doi:10.1016/S0889-8529(02)00061-0. PMID 12699289.
- ↑ 29.0 29.1 29.2 29.3 29.4 Raisz L (2005). "Pathogenesis of osteoporosis: concepts, conflicts, and prospects". J Clin Invest. 115 (12): 3318–25. doi:10.1172/JCI27071. PMC 1297264. PMID 16322775. Archived from the original on 24 August 2007.
- ↑ Ojo F, Al Snih S, Ray LA, Raji MA, Markides KS (2007). "History of fractures as predictor of subsequent hip and nonhip fractures among older Mexican Americans". Journal of the National Medical Association. 99 (4): 412–18. PMC 2569658. PMID 17444431.
- ↑ 31.0 31.1 Brian K Alldredge; Koda-Kimble, Mary Anne; Young, Lloyd Y.; Wayne A Kradjan; B. Joseph Guglielmo (2009). Applied therapeutics: the clinical use of drugs. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins. pp. 101–03. ISBN 978-0-7817-6555-8.
- ↑ 32.0 32.1 Poole KE, Compston JE (December 2006). "Osteoporosis and its management". BMJ. 333 (7581): 1251–56. doi:10.1136/bmj.39050.597350.47. PMC 1702459. PMID 17170416.
- ↑ Berg KM, Kunins HV, Jackson JL, Nahvi S, Chaudhry A, Harris KA, Malik R, Arnsten JH (2008). "Association between alcohol consumption and both osteoporotic fracture and bone density". Am J Med. 121 (5): 406–18. doi:10.1016/j.amjmed.2007.12.012. PMC 2692368. PMID 18456037.
- ↑ Nieves JW (2005). "Osteoporosis: the role of micronutrients". Am J Clin Nutr. 81 (5): 1232S–39S. doi:10.1093/ajcn/81.5.1232. PMID 15883457.
- ↑ Gielen E, Boonen S, Vanderschueren D, Sinnesael M, Verstuyf A, Claessens F, Milisen K, Verschueren S (2011). "Calcium and vitamin d supplementation in men". Journal of Osteoporosis. 2011: 1–6. doi:10.4061/2011/875249. PMC 3163142. PMID 21876835.
- ↑ Wong PK, Christie JJ, Wark JD (2007). "The effects of smoking on bone health". Clin. Sci. 113 (5): 233–41. doi:10.1042/CS20060173. PMID 17663660.
- ↑ Ilich JZ, Kerstetter JE (2000). "Nutrition in Bone Health Revisited: A Story Beyond Calcium". Journal of the American College of Nutrition. 19 (6): 715–37. doi:10.1080/07315724.2000.10718070. PMID 11194525. Archived from the original on 7 August 2009. Retrieved 6 October 2009.
- ↑ Weiss LA, Barrett-Connor E, von Mühlen D (2005). "Ratio of n−6 to n−3 fatty acids and bone mineral density in older adults: the Rancho Bernardo Study". Am J Clin Nutr. 81 (4): 934–38. doi:10.1093/ajcn/81.4.934. PMID 15817874.
- ↑ Abelow BJ, Holford TR, Insogna KL (1992). "Cross-cultural association between dietary animal protein and hip fracture: a hypothesis". Calcified Tissue International. 50 (1): 14–18. CiteSeerX 10.1.1.674.9378. doi:10.1007/BF00297291. PMID 1739864. S2CID 25002614.
- ↑ Hegsted M, Schuette SA, Zemel MB, Linkswiler HM (1981). "Urinary calcium and calcium balance in young men as affected by level of protein and phosphorus intake". The Journal of Nutrition. 111 (3): 553–62. doi:10.1093/jn/111.3.553. PMID 7205408.
- ↑ Kerstetter JE, Allen LH (1990). "Dietary protein increases urinary calcium". Journal of Nutrition. 120 (1): 134–36. doi:10.1093/jn/120.1.134. PMID 2406396.
- ↑ Feskanich D, Willett WC, Stampfer MJ, Colditz GA (1996). "Protein consumption and bone fractures in women". Am. J. Epidemiol. 143 (5): 472–79. doi:10.1093/oxfordjournals.aje.a008767. PMID 8610662.
- ↑ Kerstetter JE, Kenny AM, Insogna KL (2011). "Dietary protein and skeletal health: A review of recent human research". Current Opinion in Lipidology. 22 (1): 16–20. doi:10.1097/MOL.0b013e3283419441. PMC 4659357. PMID 21102327.
- ↑ Bonjour JP (2005). "Dietary protein: An essential nutrient for bone health". Journal of the American College of Nutrition. 24 (6 Suppl): 526S–36S. doi:10.1080/07315724.2005.10719501. PMID 16373952.
- ↑ Kerstetter JE, O'Brien KO, Insogna KL (2003). "Dietary protein, calcium metabolism, and skeletal homeostasis revisited". Am. J. Clin. Nutr. 78 (3 Suppl): 584S–92S. doi:10.1093/ajcn/78.3.584S. PMID 12936953.
- ↑ Schönau E, Werhahn E, Schiedermaier U, Mokow E, Schiessl H, Scheidhauer K, Michalk D (1996). "Influence of muscle strength on bone strength during childhood and adolescence". Hormone Research. 45 (Suppl. 1): 63–66. doi:10.1159/000184834. PMID 8805035.
- ↑ Shapses SA, Riedt CS (1 June 2006). "Bone, body weight, and weight reduction: what are the concerns?". J. Nutr. 136 (6): 1453–56. doi:10.1093/jn/136.6.1453. PMC 4016235. PMID 16702302.
- ↑ Pollock N, Grogan C, Perry M, Pedlar C, Cooke K, Morrissey D, Dimitriou L (2010). "Bone-mineral density and other features of the female athlete triad in elite endurance runners: A longitudinal and cross-sectional observational study". International Journal of Sport Nutrition and Exercise Metabolism. 20 (5): 418–26. doi:10.1123/ijsnem.20.5.418. PMID 20975110. S2CID 5867410.
- ↑ Gibson JH, Mitchell A, Harries MG, Reeve J (2004). "Nutritional and exercise-related determinants of bone density in elite female runners". Osteoporosis International. 15 (8): 611–18. doi:10.1007/s00198-004-1589-2. PMID 15048548. S2CID 42115482.
- ↑ Hetland ML, Haarbo J, Christiansen C (1993). "Low bone mass and high bone turnover in male long distance runners". The Journal of Clinical Endocrinology and Metabolism. 77 (3): 770–75. doi:10.1210/jcem.77.3.8370698. PMID 8370698.
- ↑ Brahm H, Ström H, Piehl-Aulin K, Mallmin H, Ljunghall S (1997). "Bone metabolism in endurance trained athletes: A comparison to population-based controls based on DXA, SXA, quantitative ultrasound, and biochemical markers". Calcified Tissue International. 61 (6): 448–54. doi:10.1007/s002239900366. PMID 9383270. S2CID 32005973.
- ↑ MacKelvie KJ, Taunton JE, McKay HA, Khan KM (2000). "Bone mineral density and serum testosterone in chronically trained, high mileage 40–55 year old male runners". British Journal of Sports Medicine. 34 (4): 273–78. doi:10.1136/bjsm.34.4.273. PMC 1724199. PMID 10953900.
- ↑ Staessen JA, Roels HA, Emelianov D, Kuznetsova T, Thijs L, Vangronsveld J, Fagard R (1999). "Environmental exposure to cadmium, forearm bone density, and risk of fractures: prospective population study. Public Health and Environmental Exposure to Cadmium (PheeCad) Study Group". Lancet. 353 (9159): 1140–44. doi:10.1016/S0140-6736(98)09356-8. PMID 10209978. S2CID 33697569.
- ↑ Tucker KL, Morita K, Qiao N, Hannan MT, Cupples LA, Kiel DP (2006). "Colas, but not other carbonated beverages, are associated with low bone mineral density in older women: The Framingham Osteoporosis Study". Am. J. Clin. Nutr. 84 (4): 936–42. doi:10.1093/ajcn/84.4.936. PMID 17023723.
- ↑ American Academy of Pediatrics Committee on School Health (2004). "Soft drinks in schools". Pediatrics. 113 (1 Pt 1): 152–54. doi:10.1542/peds.113.1.152. PMID 14702469.
- ↑ Zhou B, Huang Y, Li H, Sun W, Liu J (January 2016). "Proton-pump inhibitors and risk of fractures: an update meta-analysis". Osteoporosis International. 27 (1): 339–47. doi:10.1007/s00198-015-3365-x. PMID 26462494. S2CID 13532091.
- ↑ 57.0 57.1 57.2 57.3 57.4 Simonelli, C; et al. (July 2006). "ICSI Health Care Guideline: Diagnosis and Treatment of Osteoporosis, 5th edition". Institute for Clinical Systems Improvement. Archived from the original (PDF) on 18 July 2007. Retrieved 8 April 2008.
- ↑ 58.00 58.01 58.02 58.03 58.04 58.05 58.06 58.07 58.08 58.09 58.10 58.11 Kohlmeier, Lynn Kohlmeier (1998). "Osteoporosis – Risk Factors, Screening, and Treatment". Medscape Portals. Archived from the original on 19 December 2008. Retrieved 11 May 2008.
- ↑ 59.0 59.1 59.2 Ebeling PR (2008). "Clinical practice. Osteoporosis in men". N Engl J Med. 358 (14): 1474–82. doi:10.1056/NEJMcp0707217. PMID 18385499.
- ↑ 60.0 60.1 Mirza F, Canalis E (September 2015). "Management of endocrine disease: Secondary osteoporosis: pathophysiology and management". Eur J Endocrinol (Review). 173 (3): R131–51. doi:10.1530/EJE-15-0118. PMC 4534332. PMID 25971649.
- ↑ 61.0 61.1 61.2 Henwood MJ, Binkovitz L (2009). "Update on pediatric bone health". The Journal of the American Osteopathic Association. 109 (1): 5–12. PMID 19193819. Archived from the original on 4 March 2016. Retrieved 23 April 2013.
- ↑ Beto JA (January 2015). "The role of calcium in human aging". Clin Nutr Res (Review). 4 (1): 1–8. doi:10.7762/cnr.2015.4.1.1. PMC 4337919. PMID 25713787.
- ↑ Mornet, Etienne; Nunes, Mark E (20 November 2007). "Hypophosphatasia". GeneReviews: Hypophostasia. NCBI. Archived from the original on 18 January 2017.
- ↑ "Hypophosphatasia Case Studies: Dangers of Misdiagnosis". Hypophosphatasia.com. Archived from the original on 8 August 2014. Retrieved 5 August 2014.
- ↑ Invernizzi M, Carda S, Viscontini GS, Cisari C (2009). "Osteoporosis in Parkinson's disease". Parkinsonism & Related Disorders. 15 (5): 339–46. doi:10.1016/j.parkreldis.2009.02.009. PMID 19346153.
- ↑ Celsi F, Pizzo P, Brini M, Leo S, Fotino C, Pinton P, Rizzuto R (2009). "Mitochondria, calcium and cell death: A deadly triad in neurodegeneration". Biochimica et Biophysica Acta (BBA) - Bioenergetics. 1787 (5): 335–44. doi:10.1016/j.bbabio.2009.02.021. PMC 2696196. PMID 19268425.
- ↑ Bone and Tooth Society of Great Britain; National Osteoporosis Society; Royal College of Physicians (2003). Glucocorticoid-induced Osteoporosis (PDF). London, UK: Royal College of Physicians of London. ISBN 978-1-86016-173-5. Archived from the original (PDF) on 14 January 2012. Retrieved 3 October 2011.
- ↑ Homik J, Suarez-Almazor ME, Shea B, Cranney A, Wells G, Tugwell P (27 April 1998). "Calcium and vitamin D for corticosteroid-induced osteoporosis". The Cochrane Database of Systematic Reviews (2): CD000952. doi:10.1002/14651858.cd000952. PMC 7046131. PMID 10796394.
- ↑ Gourlay M, Franceschini N, Sheyn Y (February 2007). "Prevention and treatment strategies for glucocorticoid-induced osteoporotic fractures". Clinical Rheumatology. 26 (2): 144–53. doi:10.1007/s10067-006-0315-1. PMID 16670825. S2CID 26017061.
- ↑ Petty SJ, O'Brien TJ, Wark JD (2007). "Anti-epileptic medication and bone health". Osteoporosis International. 18 (2): 129–42. doi:10.1007/s00198-006-0185-z. PMID 17091219. S2CID 2953573.
- ↑ Ruiz-Irastorza G, Khamashta MA, Hughes GR (2002). "Heparin and osteoporosis during pregnancy: 2002 update". Lupus. 11 (10): 680–82. doi:10.1191/0961203302lu262oa. PMID 12413068. S2CID 2922860.
- ↑ Gage BF, Birman-Deych E, Radford MJ, Nilasena DS, Binder EF (2006). "Risk of osteoporotic fracture in elderly patients taking warfarin: results from the National Registry of Atrial Fibrillation 2". Arch. Intern. Med. 166 (2): 241–46. doi:10.1001/archinte.166.2.241. PMID 16432096.
- ↑ Yang YX, Lewis JD, Epstein S, Metz DC (2006). "Long-term proton pump inhibitor therapy and risk of hip fracture". JAMA. 296 (24): 2947–53. doi:10.1001/jama.296.24.2947. PMID 17190895.
- ↑ Murphy CE, Rodgers PT (2007). "Effects of thiazolidinediones on bone loss and fracture". Annals of Pharmacotherapy. 41 (12): 2014–18. doi:10.1345/aph.1K286. PMID 17940125. S2CID 21577063.
- ↑ 75.0 75.1 Latimer B (2005). "The perils of being bipedal". Ann Biomed Eng. 33 (1): 3–6. doi:10.1007/s10439-005-8957-8. PMID 15709701. S2CID 43294733.
- ↑ 76.0 76.1 76.2 Cotter M; et al. (2011). "Human evolution and osteoporosis-related spinal fractures". PLOS ONE. 6 (10): e26658. Bibcode:2011PLoSO...626658C. doi:10.1371/journal.pone.0026658. PMC 3197574. PMID 22028933.
- ↑ Eaton SB, Nelson DA (1991). "Calcium in evolutionary perspective". Am. J. Clin. Nutr. 54 (1 Suppl): 281S–87S. doi:10.1093/ajcn/54.1.281S. PMID 2053574.
- ↑ Frost HM, Thomas CC. Bone Remodeling Dynamics. Springfield, IL: 1963.
- ↑ Wu S, Liu Y, Zhang L, Han Y, Lin Y, Deng HW (2013). "Genome-wide approaches for identifying genetic risk factors for osteoporosis". Genome Medicine. 5 (5): 44. doi:10.1186/gm448. PMC 3706967. PMID 23731620.
- ↑ Paccou J, Hardouin P, Cotten A, Penel G, Cortet B (October 2015). "The Role of Bone Marrow Fat in Skeletal Health: Usefulness and Perspectives for Clinicians". The Journal of Clinical Endocrinology and Metabolism. 100 (10): 3613–21. doi:10.1210/jc.2015-2338. PMID 26244490.
- ↑ 81.0 81.1 81.2 Guglielmi G, Scalzo G (6 May 2010). "Imaging tools transform diagnosis of osteoporosis". Diagnostic Imaging Europe. 26: 7–11. Archived from the original on 2 June 2010.
- ↑ Leib ES, Lewiecki EM, Binkley N, Hamdy RC (2004). "Official positions of the International Society for Clinical Densitometry". J Clin Densitom. 7 (1): 1–5. doi:10.1385/JCD:7:1:1. PMID 14742881. quoted in: "Diagnosis of osteoporosis in men, premenopausal women, and children" Archived 24 February 2008 at the Wayback Machine
- ↑ Yasuda Y, Kaleta J, Brömme D (2005). "The role of cathepsins in osteoporosis and arthritis: rationale for the design of new therapeutics". Adv. Drug Deliv. Rev. 57 (7): 973–93. doi:10.1016/j.addr.2004.12.013. PMID 15876399.
- ↑ Meunier, Pierre (1998). Osteoporosis: Diagnosis and Management. London: Taylor and Francis. ISBN 978-1-85317-412-4.
- ↑ Bindex, a Radiation-Free Device for Osteoporosis Screening, FDA Cleared. May 2016 Archived 15 June 2016 at the Wayback Machine
- ↑ 86.0 86.1 86.2 Curry SJ, Krist AH, Owens DK, Barry MJ, Caughey AB, Davidson KW, Doubeni CA, Epling JW, Kemper AR, Kubik M, Landefeld CS, Mangione CM, Phipps MG, Pignone M, Silverstein M, Simon MA, Tseng CW, Wong JB (June 2018). "Screening for Osteoporosis to Prevent Fractures: US Preventive Services Task Force Recommendation Statement". JAMA. 319 (24): 2521–2531. doi:10.1001/jama.2018.7498. PMID 29946735.
- ↑ U.S. Preventive Services Task Force (March 2011). "Screening for osteoporosis: U.S. preventive services task force recommendation statement". Annals of Internal Medicine. 154 (5): 356–64. doi:10.7326/0003-4819-154-5-201103010-00307. PMID 21242341.
- ↑ "100 most recent Archives 2017 2016 2015 2014 2013 2012 2011 2010 2009 2008 2007 2006 2005 Bone fragility: preventing fractures". Prescrire International. 26 (181): 103–06. April 2017. Archived from the original on 8 September 2017.
- ↑ International Society for Clinical Densitometry (ISCD). 2013 ISCD Official Positions – Adult. (2013). at "2013 ISCD Official Positions - Adult - International Society for Clinical Densitometry (ISCD)". Archived from the original on 5 May 2015. Retrieved 4 May 2015.
- ↑ Rud B, Hilden J, Hyldstrup L, Hróbjartsson A (April 2009). "The Osteoporosis Self-Assessment Tool versus alternative tests for selecting postmenopausal women for bone mineral density assessment: a comparative systematic review of accuracy". Osteoporosis International. 20 (4): 599–607. doi:10.1007/s00198-008-0713-0. PMID 18716823. S2CID 13641749.
- ↑ Ebeling PR, Daly RM, Kerr DA, Kimlin MG (October 2013). "Building healthy bones throughout life: an evidence-informed strategy to prevent osteoporosis in Australia" (PDF). The Medical Journal of Australia. 199 (7 Suppl): 90–91. doi:10.5694/mja12.11363. hdl:10536/DRO/DU:30060407. PMID 25370432. Archived (PDF) from the original on 6 August 2020. Retrieved 2 August 2020.
- ↑ 92.0 92.1 92.2 92.3 92.4 92.5 92.6 92.7 92.8 Body JJ (2011). "How to manage postmenopausal osteoporosis?". Acta Clinica Belgica. 66 (6): 443–7. doi:10.1179/ACB.66.6.2062612. PMID 22338309.
- ↑ 93.0 93.1 Ludvigsson JF, Bai JC, Biagi F, Card TR, Ciacci C, Ciclitira PJ, Green PH, Hadjivassiliou M, Holdoway A, van Heel DA, Kaukinen K, Leffler DA, Leonard JN, Lundin KE, McGough N, Davidson M, Murray JA, Swift GL, Walker MM, Zingone F, Sanders DS, BSG Coeliac Disease Guidelines Development Group, British Society of Gastroenterology (August 2014). "Diagnosis and management of adult coeliac disease: guidelines from the British Society of Gastroenterology". Gut (Review). 63 (8): 1210–28. doi:10.1136/gutjnl-2013-306578. PMC 4112432. PMID 24917550.
- ↑ Balk EM, Adam GP, Langberg VN, Earley A, Clark P, Ebeling PR, Mithal A, Rizzoli R, Zerbini CA, Pierroz DD, Dawson-Hughes B (December 2017). "Global dietary calcium intake among adults: a systematic review". Osteoporosis International. 28 (12): 3315–3324. doi:10.1007/s00198-017-4230-x. PMC 5684325. PMID 29026938.
- ↑ Wahl DA, Cooper C, Ebeling PR, Eggersdorfer M, Hilger J, Hoffmann K, et al. (29 August 2012). "A global representation of vitamin D status in healthy populations". Archives of Osteoporosis. 7 (1–2): 155–72. doi:10.1007/s11657-012-0093-0. hdl:11343/220606. PMID 23225293. S2CID 207300035.
- ↑ Wahl DA, Cooper C, Ebeling PR, Eggersdorfer M, Hilger J, Hoffmann K, et al. (1 February 2013). "A global representation of vitamin D status in healthy populations: reply to comment by Saadi". Archives of Osteoporosis. 8 (1–2): 122. doi:10.1007/s11657-013-0122-7. PMID 23371520. S2CID 5929230.
- ↑ "Drugs for Postmenopausal Osteoporosis". The Medical Letter on Drugs and Therapeutics. 56 (1452): 91–96. 29 September 2014. PMID 25247344.
- ↑ 98.0 98.1 98.2 98.3 98.4 Kahwati LC, Weber RP, Pan H, Gourlay M, LeBlanc E, Coker-Schwimmer M, Viswanathan M (April 2018). "Vitamin D, Calcium, or Combined Supplementation for the Primary Prevention of Fractures in Community-Dwelling Adults: Evidence Report and Systematic Review for the US Preventive Services Task Force". JAMA (Systematic Review & Meta-Analysis). 319 (15): 1600–1612. doi:10.1001/jama.2017.21640. PMID 29677308. S2CID 205090176.
- ↑ "Final Recommendation Statement Vitamin D, Calcium, or Combined Supplementation for the Primary Prevention of Fractures in Community-Dwelling Adults: Preventive Medication". www.uspreventiveservicestaskforce.org. USPSTF Program Office. Archived from the original on 8 March 2020. Retrieved 2 August 2020.
- ↑ Bolland MJ, Leung W, Tai V, Bastin S, Gamble GD, Grey A, Reid IR (September 2015). "Calcium intake and risk of fracture: systematic review". BMJ. 351: h4580. doi:10.1136/bmj.h4580. PMC 4784799. PMID 26420387.
- ↑ DIPART Group (January 2010). "Patient level pooled analysis of 68 500 patients from seven major vitamin D fracture trials in US and Europe". BMJ. 340: b5463. doi:10.1136/bmj.b5463. PMC 2806633. PMID 20068257.
- ↑ 102.0 102.1 102.2 Avenell A, Mak JC, O'Connell D (April 2014). "Vitamin D and vitamin D analogues for preventing fractures in post-menopausal women and older men". The Cochrane Database of Systematic Reviews. 4 (4): CD000227. doi:10.1002/14651858.CD000227.pub4. PMC 7032685. PMID 24729336.
- ↑ Bolland MJ, Avenell A, Baron JA, Grey A, MacLennan GS, Gamble GD, Reid IR (2010). "Effect of calcium supplements on risk of myocardial infarction and cardiovascular events: meta-analysis". BMJ (Clinical Research Ed.). 341: c3691. doi:10.1136/bmj.c3691. PMC 2912459. PMID 20671013.
- ↑ Bolland MJ, Grey A, Avenell A, Gamble GD, Reid IR (2011). "Calcium supplements with or without vitamin D and risk of cardiovascular events: reanalysis of the Women's Health Initiative limited access dataset and meta-analysis". BMJ. 342: d2040. doi:10.1136/bmj.d2040. PMC 3079822. PMID 21505219.
- ↑ Hosoi T (November 2010). "Genetic aspects of osteoporosis". Journal of Bone and Mineral Metabolism. 28 (6): 601–7. doi:10.1007/s00774-010-0217-9. PMID 20697753. S2CID 35412918.
- ↑ "Preventing and Reversing Osteoporosis". Physicians Committee for Responsible Medicine. Archived from the original on 14 January 2020. Retrieved 5 August 2019.
- ↑ "Calcium: What's Best for Your Bones and Health?". The Nutrition Source. 18 September 2012. Archived from the original on 4 February 2011. Retrieved 5 August 2019.
- ↑ Laskou F, Dennison E (April 2019). "Interaction of Nutrition and Exercise on Bone and Muscle". European Endocrinology. 15 (1): 11–12. doi:10.17925/ee.2019.15.1.11. PMC 6587895. PMID 31244903.
- ↑ 109.0 109.1 109.2 109.3 109.4 Howe TE, Shea B, Dawson LJ, Downie F, Murray A, Ross C, et al. (July 2011). "Exercise for preventing and treating osteoporosis in postmenopausal women". The Cochrane Database of Systematic Reviews. Art. No.: CD000333 (7): CD000333. doi:10.1002/14651858.CD000333.pub2. PMID 21735380.
- ↑ Weaver CM, Gordon CM, Janz KF, Kalkwarf HJ, Lappe JM, Lewis R, et al. (April 2016). "The National Osteoporosis Foundation's position statement on peak bone mass development and lifestyle factors: a systematic review and implementation recommendations". Osteoporosis International. 27 (4): 1281–1386. doi:10.1007/s00198-015-3440-3. PMC 4791473. PMID 26856587.
- ↑ Gibbs, Jenna C.; MacIntyre, Norma J.; Ponzano, Matteo; Templeton, Jeffrey Alan; Thabane, Lehana; Papaioannou, Alexandra; Giangregorio, Lora M. (5 July 2019). "Exercise for improving outcomes after osteoporotic vertebral fracture". The Cochrane Database of Systematic Reviews. 7: CD008618. doi:10.1002/14651858.CD008618.pub3. ISSN 1469-493X. PMC 6609547. PMID 31273764.
- ↑ Gibbs, Jenna C; MacIntyre, Norma J; Ponzano, Matteo; Templeton, Jeffrey Alan; Thabane, Lehana; Papaioannou, Alexandra; Giangregorio, Lora M (5 July 2019). Cochrane Musculoskeletal Group (ed.). "Exercise for improving outcomes after osteoporotic vertebral fracture". Cochrane Database of Systematic Reviews. 7: CD008618. doi:10.1002/14651858.CD008618.pub3. PMC 6609547. PMID 31273764.
- ↑ Zhou, X; Deng, H; Shen, X; Lei, Q (2018). "Effect of balance training on falls in patients with osteoporosis: A systematic review and meta-analysis". Journal of Rehabilitation Medicine. 50 (7): 577–581. doi:10.2340/16501977-2334. ISSN 1650-1977. PMID 29767225.
- ↑ Gibbs, Jenna C; MacIntyre, Norma J; Ponzano, Matteo; Templeton, Jeffrey Alan; Thabane, Lehana; Papaioannou, Alexandra; Giangregorio, Lora M (5 July 2019). Cochrane Musculoskeletal Group (ed.). "Exercise for improving outcomes after osteoporotic vertebral fracture". Cochrane Database of Systematic Reviews. 7: CD008618. doi:10.1002/14651858.CD008618.pub3. PMC 6609547. PMID 31273764.
- ↑ Gibbs, Jenna C; MacIntyre, Norma J; Ponzano, Matteo; Templeton, Jeffrey Alan; Thabane, Lehana; Papaioannou, Alexandra; Giangregorio, Lora M (5 July 2019). Cochrane Musculoskeletal Group (ed.). "Exercise for improving outcomes after osteoporotic vertebral fracture". Cochrane Database of Systematic Reviews. 7: CD008618. doi:10.1002/14651858.CD008618.pub3. PMC 6609547. PMID 31273764.
- ↑ Zhou, X; Deng, H; Shen, X; Lei, Q (2018). "Effect of balance training on falls in patients with osteoporosis: A systematic review and meta-analysis". Journal of Rehabilitation Medicine. 50 (7): 577–581. doi:10.2340/16501977-2334. ISSN 1650-1977. PMID 29767225.
- ↑ Body JJ, Bergmann P, Boonen S, Boutsen Y, Bruyere O, Devogelaer JP, Goemaere S, Hollevoet N, Kaufman JM, Milisen K, Rozenberg S, Reginster JY (2011). "Non-pharmacological management of osteoporosis: a consensus of the Belgian Bone Club". Osteoporos Int. 22 (11): 2769–88. doi:10.1007/s00198-011-1545-x. PMC 3186889. PMID 21360219.
- ↑ Kasturi GC, Adler RA (2011). "Osteoporosis: nonpharmacologic management". PM&R. 3 (6): 562–72. doi:10.1016/j.pmrj.2010.12.014. PMID 21478069.
- ↑ Kim, Beomchang; Cho, Yong Jin; Lim, Wonbong (December 2021). "Osteoporosis therapies and their mechanisms of action (Review)". Experimental and Therapeutic Medicine. 22 (6): 1379. doi:10.3892/etm.2021.10815. ISSN 1792-1015.
- ↑ 120.0 120.1 Whitaker M, Guo J, Kehoe T, Benson G (May 2012). "Bisphosphonates for osteoporosis--where do we go from here?". The New England Journal of Medicine. 366 (22): 2048–51. doi:10.1056/NEJMp1202619. PMID 22571168.
- ↑ 121.0 121.1 Ward L, Tricco AC, Phuong P, Cranney A, Barrowman N, Gaboury I, et al. (October 2007). "Bisphosphonate therapy for children and adolescents with secondary osteoporosis". The Cochrane Database of Systematic Reviews (4): CD005324. doi:10.1002/14651858.cd005324.pub2. PMID 17943849.
- ↑ Cummings, Steven R.; Lui, Li-Yung; Eastell, Richard; Allen, Isabel E. (19 August 2019). "Association Between Drug Treatments for Patients With Osteoporosis and Overall Mortality Rates". JAMA Internal Medicine. 179 (11): 1491. doi:10.1001/jamainternmed.2019.2779. PMC 6704731. PMID 31424486.
- ↑ Suresh E, Pazianas M, Abrahamsen B (January 2014). "Safety issues with bisphosphonate therapy for osteoporosis". Rheumatology. 53 (1): 19–31. doi:10.1093/rheumatology/ket236. PMID 23838024.
- ↑ 124.0 124.1 Adler RA, El-Hajj Fuleihan G, Bauer DC, Camacho PM, Clarke BL, Clines GA, et al. (January 2016). "Managing Osteoporosis in Patients on Long-Term Bisphosphonate Treatment: Report of a Task Force of the American Society for Bone and Mineral Research". Journal of Bone and Mineral Research. 31 (1): 16–35. doi:10.1002/jbmr.2708. PMC 4906542. PMID 26350171.
- ↑ 125.0 125.1 125.2 Qaseem A, Forciea MA, McLean RM, Denberg TD (June 2017). "Treatment of Low Bone Density or Osteoporosis to Prevent Fractures in Men and Women: A Clinical Practice Guideline Update From the American College of Physicians". Annals of Internal Medicine. 166 (11): 818–839. doi:10.7326/M15-1361. PMID 28492856.
- ↑ "Therapeutics Initiative | [147] Screening to reduce fragility fractures: new trials, still ineffective". 28 February 2024. Archived from the original on 28 March 2024. Retrieved 24 March 2024.
- ↑ Davis S, Sachdeva A, Goeckeritz B, Oliver A (2010). "Approved treatments for osteoporosis and what's in the pipeline". Drug Benefit Trends. 22 (4): 121–24. Archived from the original on 28 July 2010.
- ↑ 128.0 128.1 Nayak S, Greenspan SL (March 2017). "Osteoporosis Treatment Efficacy for Men: A Systematic Review and Meta-Analysis". Journal of the American Geriatrics Society. 65 (3): 490–495. doi:10.1111/jgs.14668. PMC 5358515. PMID 28304090.
- ↑ Haguenauer D, Welch V, Shea B, Tugwell P, Wells G (2000). "Fluoride for treating postmenopausal osteoporosis". The Cochrane Database of Systematic Reviews (4): CD002825. doi:10.1002/14651858.CD002825. PMID 11034769.
- ↑ Vestergaard P, Jorgensen NR, Schwarz P, Mosekilde L (March 2008). "Effects of treatment with fluoride on bone mineral density and fracture risk--a meta-analysis". Osteoporosis International. 19 (3): 257–68. doi:10.1007/s00198-007-0437-6. PMID 17701094. S2CID 25890845.
- ↑ Han SL, Wan SL (February 2012). "Effect of teriparatide on bone mineral density and fracture in postmenopausal osteoporosis: meta-analysis of randomised controlled trials". International Journal of Clinical Practice. 66 (2): 199–209. doi:10.1111/j.1742-1241.2011.02837.x. PMID 22257045.
- ↑ O'Donnell S, Cranney A, Wells GA, Adachi JD, Reginster JY (October 2006). Cranney A (ed.). "Strontium ranelate for preventing and treating postmenopausal osteoporosis". The Cochrane Database of Systematic Reviews (4): CD005326. doi:10.1002/14651858.CD005326.pub3. PMID 17054253.
- ↑ "Background Document for Meeting of Advisory Committee for Reproductive Health Drugs and Drug Safety and Risk Management Advisory Committee" (PDF). FDA. March 2013. Archived (PDF) from the original on 9 June 2013.
- ↑ "Osteoporosis – primary prevention (TA160) : Alendronate, etidronate, risedronate, raloxifene and strontium ranelate for the primary prevention of osteoporotic fragility fractures in postmenopausal women". UK: National Institute for Health and Care Excellence (NICE). January 2011. Archived from the original on 22 October 2013.
- ↑ Liu, Yunxia; Liu, Jian Ping; Xia, Yun (6 March 2014). "Chinese herbal medicines for treating osteoporosis". Cochrane Database of Systematic Reviews (3): CD005467. doi:10.1002/14651858.cd005467.pub2. ISSN 1465-1858. PMID 24599707.
- ↑ Cranney A, Jamal SA, Tsang JF, Josse RG, Leslie WD (2007). "Low bone mineral density and fracture burden in postmenopausal women". CMAJ. 177 (6): 575–80. doi:10.1503/cmaj.070234. PMC 1963365. PMID 17846439.
- ↑ Hannan EL, Magaziner J, Wang JJ, Eastwood EA, Silberzweig SB, Gilbert M, Morrison RS, McLaughlin MA, Orosz GM, Siu AL (2001). "Mortality and locomotion 6 months after hospitalization for hip fracture: risk factors and risk-adjusted hospital outcomes". JAMA. 285 (21): 2736–42. doi:10.1001/jama.285.21.2736. PMID 11386929.
- ↑ Brenneman SK, Barrett-Connor E, Sajjan S, Markson LE, Siris ES (2006). "Impact of recent fracture on health-related quality of life in postmenopausal women". J. Bone Miner. Res. 21 (6): 809–16. doi:10.1359/jbmr.060301. PMID 16753011.
- ↑ 139.0 139.1 Riggs BL, Melton LJ (1995). "The worldwide problem of osteoporosis: insights afforded by epidemiology". Bone. 17 (5 Suppl): 505S–11S. doi:10.1016/8756-3282(95)00258-4. PMID 8573428.
- ↑ 140.0 140.1 "MerckMedicus Modules: Osteoporosis – Epidemiology". Merck & Co., Inc. Archived from the original on 28 December 2007. Retrieved 13 June 2008.
- ↑ Cauley JA, Hochberg MC, Lui LY, Palermo L, Ensrud KE, Hillier TA, Nevitt MC, Cummings SR (2007). "Long-term risk of incident vertebral fractures". JAMA. 298 (23): 2761–67. doi:10.1001/jama.298.23.2761. PMID 18165669.
- ↑ 142.0 142.1 142.2 Kanis JA, Odén A, McCloskey EV, Johansson H, Wahl DA, Cooper C (September 2012). "A systematic review of hip fracture incidence and probability of fracture worldwide". Osteoporosis International. 23 (9): 2239–56. doi:10.1007/s00198-012-1964-3. PMC 3421108. PMID 22419370.
- ↑ International Osteoporosis Foundation. Epidemiology Archived 9 August 2015 at the Wayback Machine.
- ↑ 144.0 144.1 144.2 Ji MX, Yu Q (March 2015). "Primary osteoporosis in postmenopausal women". Chronic Diseases and Translational Medicine. 1 (1): 9–13. doi:10.1016/j.cdtm.2015.02.006. PMC 5643776. PMID 29062981.
- ↑ 145.0 145.1 145.2 "The Global Burden of Osteoporosis | International Osteoporosis Foundation". www.iofbonehealth.org. Archived from the original on 5 March 2016. Retrieved 9 February 2016.
- ↑ 146.0 146.1 146.2 Cauley JA (July 2011). "Defining ethnic and racial differences in osteoporosis and fragility fractures". Clinical Orthopaedics and Related Research. 469 (7): 1891–9. doi:10.1007/s11999-011-1863-5. PMC 3111798. PMID 21431462.
- ↑ Herrmann M, Peter Schmidt J, Umanskaya N, Wagner A, Taban-Shomal O, Widmann T, Colaianni G, Wildemann B, Herrmann W (2007). "The role of hyperhomocysteinemia as well as folate, vitamin B(6) and B(12) deficiencies in osteoporosis: a systematic review". Clinical Chemistry and Laboratory Medicine. 45 (12): 1621–32. doi:10.1515/cclm.2007.362. PMID 18067447.
- ↑ Ganji R, Moghbeli M, Sadeghi R, Bayat G, Ganji A (February 2019). "Prevalence of osteoporosis and osteopenia in men and premenopausal women with celiac disease: a systematic review". Nutrition Journal. 18 (1): 9. doi:10.1186/s12937-019-0434-6. PMC 6504166. PMID 30732599.
- ↑ Ganji R, Moghbeli M, Sadeghi R, Bayat G, Ganji A (February 2019). "Prevalence of osteoporosis and osteopenia in men and premenopausal women with celiac disease: a systematic review". Nutrition Journal. 18 (1): 9. doi:10.1186/s12937-019-0434-6. PMC 6504166. PMID 30732599.
- ↑ "What People With Celiac Disease Need To Know About Osteoporosis | NIH Osteoporosis and Related Bone Diseases National Resource Center". www.bones.nih.gov. Archived from the original on 6 August 2020. Retrieved 1 August 2019.
- ↑ Gerald N. Grob (2014). Aging Bones: A Short History of Osteoporosis. Johns Hopkins UP. p. 5. ISBN 9781421413181. Archived from the original on 23 July 2014.
- ↑ Albright F, Bloomberg E, Smith PH (1940). "Postmenopausal osteoporosis". Trans. Assoc. Am. Physicians. 55: 298–305.
- ↑ Patlak M (2001). "Bone builders: the discoveries behind preventing and treating osteoporosis". FASEB J. 15 (10): 1677E–E. doi:10.1096/fj.15.10.1677e. PMID 11481214.
- ↑ Hirata K, Morimoto I (1994). "Vertebral Osteoporosis in Late Edo Japanese". Anthropological Science. 102 (4): 345–61. doi:10.1537/ase.102.345.
External links
| Classification | |
|---|---|
| External resources |
- Osteoporosis at Curlie
- Handout on Health: Osteoporosis Archived 17 August 2017 at the Wayback Machine – US National Institute of Arthritis and Musculoskeletal and Skin Diseases
- Osteoporosis Archived 30 July 2017 at the Wayback Machine – l NIH Osteoporosis and Related Bone Diseases – National Resource Center
- Office of the Surgeon General (2004). Bone Health and Osteoporosis: A Report of the Surgeon General. Rockville, MD: U.S. Department of Health and Human Services. PMID 20945569. Archived from the original on 28 March 2019. Retrieved 18 July 2016.
- "Osteoporosis". MedlinePlus. U.S. National Library of Medicine. Archived from the original on 29 July 2020. Retrieved 2 August 2020.
- Pages with script errors
- Webarchive template wayback links
- CS1: long volume value
- Use dmy dates from March 2018
- Articles with invalid date parameter in template
- All articles with unsourced statements
- Articles with unsourced statements from September 2007
- Articles with unsourced statements from November 2014
- Articles with unsourced statements from March 2020
- Articles with Curlie links
- Aging-associated diseases
- Endocrine diseases
- Osteopathies
- RTT
- RTTEM
- RTTWH