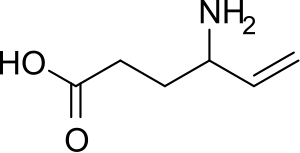
Vigabatrin
 | |
 | |
| Names | |
|---|---|
| Trade names | Sabril, Vigadrone, Kigabeq, others |
| Other names | γ-Vinyl-GABA |
| |
| Clinical data | |
| Drug class | Anticonvulsant[1] |
| Main uses | Epilepsy[1] |
| Side effects | Permanent vision problems, headache, tiredness, abdominal pain, swelling[1] |
| Pregnancy category |
|
| Routes of use | By mouth |
| Typical dose | 0.5 to 1.5 grams BID (adults)[1] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a610016 |
| Legal | |
| Legal status | |
| Pharmacokinetics | |
| Bioavailability | 80–90% |
| Protein binding | 0% |
| Metabolism | not metabolized |
| Elimination half-life | 5–8 hours in young adults, 12–13 hours in the elderly. |
| Excretion | Kidney |
| Chemical and physical data | |
| Formula | C6H11NO2 |
| Molar mass | 129.159 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 171 to 177 °C (340 to 351 °F) |
| |
| |
Vigabatrin, sold under the brand name Sabril, is a medication used to treat epilepsy.[1] Specifically it is used for complex partial seizures that are uncontrolled with other measures or for infantile spasms.[1] It is taken by mouth.[1]
Common side effects include headache, tiredness, abdominal pain, vision problems, and swelling.[1] Permanent vision problems occur in about a third of people; with an onset of a month to years after starting treatment.[2][1] It is believed to work by decreasing the breakdown of γ-aminobutyric acid (GABA).[1]
Vigabatrin was approved for medical use in the United States in 2009.[1] It became available as a generic medication in 2019.[3] In the United Kingdom a hundred tablets of 500 mg costs the NHS about £45 as of 2021.[2] In the United States this amount costs about 10,600 USD.[4] In Canada this amount costs about 96 CAD.[5]
Medical uses
Epilepsy
In Canada, vigabatrin is approved for use with other medications in treatment resistant epilepsy, complex partial seizures, secondary generalized seizures, and for monotherapy use in infantile spasms in West syndrome.[6]
As of 2003, vigabatrin is approved in Mexico for the treatment of epilepsy that is not satisfactorily controlled by conventional therapy (adjunctive or monotherapy) or in recently diagnosed patients who have not tried other agents (monotherapy).[7]
Vigabatrin is also indicated for monotherapy use in secondarily generalized tonic-clonic seizures, partial seizures, and in infantile spasms due to West syndrome.[7]
In the USA it is indicated as monotherapy for children one month to two years of age with infantile spasms for whom the potential benefits outweigh the potential risk of vision loss, and as add-on therapy for adults with refractory complex partial seizures (CPS) who have inadequately responded to several alternative treatments and for whom the potential benefits outweigh the risk of vision loss.[8]
In 1994, Feucht and Brantner-Inthaler reported that vigabatrin reduced seizures by 50-100% in 85% of children with Lennox-Gastaut syndrome who had poor results with sodium valproate.[9]
Others
Vigabatrin reduced cholecystokinin tetrapeptide-induced symptoms of panic disorder, in addition to elevated cortisol and ACTH levels, in healthy volunteers.[10]
Vigabatrin is also used to treat seizures in succinic semialdehyde dehydrogenase deficiency (SSADHD), which is an inborn GABA metabolism defect that causes intellectual disability, hypotonia, seizures, speech disturbance, and ataxia through the accumulation of γ-Hydroxybutyric acid (GHB). Vigabatrin helps lower GHB levels through GABA transaminase inhibition. However, this is in the brain only; it has no effect on peripheral GABA transaminase, so the GHB keeps building up and eventually reaches the brain.[11]
Dosage
In adults the initial dose is 500 mg twice per day.[1] This may be gradually increased up to 1.5 grams twice per day.[1]
Side effects
Central nervous system
Sleepiness (12.5%), headache (3.8%), dizziness (3.8%), nervousness (2.7%), depression (2.5%), memory disturbances (2.3%), diplopia (2.2%), aggression (2.0%), ataxia (1.9%), vertigo (1.9%), hyperactivity (1.8%), vision loss (1.6%) (See below), confusion (1.4%), insomnia (1.3%), impaired concentration (1.2%), personality issues (1.1%).[6] Out of 299 children, 33 (11%) became hyperactive.[6]
Some patients develop psychosis during the course of vigabatrin therapy,[12] which is more common in adults than in children.[13] This can happen even in patients with no prior history of psychosis.[14] Other rare CNS side effects include anxiety, emotional lability, irritability, tremor, abnormal gait, and speech disorder.[6]
Gastrointestinal
Abdominal pain (1.6%), constipation (1.4%), vomiting (1.4%), and nausea (1.4%). Dyspepsia and increased appetite occurred in less than 1% of subjects in clinical trials.[6]
Body as a whole
Fatigue (9.2%), weight gain (5.0%), asthenia (1.1%).[6]
Pregnancy
A teratology study conducted in rabbits found that a dose of 150 mg/kg/day caused cleft palate in 2% of pups and a dose of 200 mg/kg/day caused it in 9%.[6] This may be due to a decrease in methionine levels, according to a study published in March 2001.[15] In 2005, a study conducted at the University of Catania was published stating that rats whose mothers had consumed 250–1000 mg/kg/day had poorer performance in the water maze and open-field tasks, rats in the 750-mg group were underweight at birth and did not catch up to the control group, and rats in the 1000 mg group did not survive pregnancy.[16]
There is no controlled teratology data in humans to date.
Sensory
In 2003, vigabatrin was shown by Frisén and Malmgren to cause irreversible diffuse atrophy of the retinal nerve fiber layer in a retrospective study of 25 patients.[17] This has the most effect on the outer area (as opposed to the macular, or central area) of the retina.[18] Visual field defects had been reported as early as 1997 by Tom Eke and others, in the UK. Some authors, including Comaish et al. believe that visual field loss and electrophysiological changes may be demonstrable in up to 50% of Vigabatrin users.
The retinal toxicity of vigabatrin can be attributed to a taurine depletion.[19]
Due to safety issues, the Vigabatrin REMS Program is required by the FDA to ensure informed decisions before initiating and to ensure appropriate use of this drug.[20]
Interactions
A study published in 2002 found that vigabatrin causes a statistically significant increase in plasma clearance of carbamazepine.[21]
In 1984, Drs Rimmer and Richens at the University of Wales reported that administering vigabatrin with phenytoin lowered the serum phenytoin concentration in patients with treatment-resistant epilepsy.[22] Five years later, the same two scientists reported a fall in concentration of phenytoin of 23% within five weeks in a paper describing their failed attempt at elucidating the mechanism behind this interaction.[23]
Pharmacology

Vigabatrin is an irreversible mechanism-based inhibitor of gamma-aminobutyric acid aminotransferase (GABA-AT), the enzyme responsible for the catabolism of GABA. Inhibition of GABA-AT results in increased levels of GABA in the brain.[6][25] Vigabatrin is a racemic compound, and its [S]-enantiomer is pharmacologically active.[26],[27]
Pharmacokinetics
With most drugs, elimination half-life is a useful predictor of dosing schedules and the time needed to reach steady state concentrations. In the case of vigabatrin, however, it has been found that the half-life of biologic activity is far longer than the elimination half-life.[28]
For vigabatrin, there is no range of target concentrations because researchers found no difference between the serum concentration levels of responders and those of non-responders.[29] Instead, the duration of action is believed to be more a function of the GABA-T resynthesis rate; levels of GABA-T do not usually return to their normal state until six days after stopping the medication.[27]
History
Vigabatrin was developed in the 1980s with the specific goal of increasing GABA concentrations in the brain in order to stop an epileptic seizure. To do this, the drug was designed to irreversibly inhibit the GABA transaminase, which degrades the GABA substrate.
It was approved for treatment in the United Kingdom in 1989, and the United States in 2009. It was delayed in the US in 1983 because animal trials produced intramyelinic edema, however, the effects were not apparent in human trials so the drug design continued. In 1997, the trials were temporarily suspended because it was linked to peripheral visual field defects.[30]
Society and culture
Brand names
Vigabatrin is sold as Sabril in Canada,[31] Mexico,[7] and the United Kingdom.[32] The brand name in Denmark is Sabrilex. Sabril was approved in the United States on August 21, 2009 and is marketed in the U.S. by Lundbeck Inc., which acquired Ovation Pharmaceuticals, the U.S. sponsor in March 2009.
Generics
On January 16, 2019, the Food and Drug Administration (FDA) approved the first generic version of vigabatrin in the United States.[33]
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 "Vigabatrin Monograph for Professionals". Drugs.com. Archived from the original on 4 March 2021. Retrieved 4 August 2021.
- ↑ 2.0 2.1 BNF (80 ed.). BMJ Group and the Pharmaceutical Press. September 2020 – March 2021. p. 349. ISBN 978-0-85711-369-6.
{{cite book}}: CS1 maint: date format (link) - ↑ "Press Announcements - FDA approves first generic version of Sabril to help treat seizures in adults and pediatric patients with epilepsy". www.fda.gov. Archived from the original on 27 January 2019. Retrieved 21 January 2019.
- ↑ "Vigabatrin Prices, Coupons & Patient Assistance Programs". Drugs.com. Retrieved 15 September 2021.
- ↑ "Formulary Search - DIN/PIN/NPN Detail". www.formulary.health.gov.on.ca. Archived from the original on 22 September 2021. Retrieved 15 September 2021.
- ↑ 6.0 6.1 6.2 6.3 6.4 6.5 6.6 6.7 Long, Phillip W. "Vigabatrin." Archived April 23, 2006, at the Wayback Machine Internet Mental Health. 1995–2003.
- ↑ 7.0 7.1 7.2 DEF Mexico: Sabril Archived September 14, 2005, at the Wayback Machine Diccionario de Especialdades Farmaceuticas. Edicion 49, 2003.
- ↑ Bresnahan, Rebecca; Gianatsi, Myrsini; Maguire, Melissa J.; Tudur Smith, Catrin; Marson, Anthony G. (July 30, 2020). "Vigabatrin add-on therapy for drug-resistant focal epilepsy". The Cochrane Database of Systematic Reviews. 2020 (7): CD007302. doi:10.1002/14651858.CD007302.pub3. ISSN 1469-493X. PMC 8211760. PMID 32730657. Archived from the original on May 19, 2021. Retrieved July 29, 2021.
{{cite journal}}: CS1 maint: PMC embargo expired (link) - ↑ Feucht M, Brantner-Inthaler S (1994). "Gamma-vinyl-GABA (vigabatrin) in the therapy of Lennox-Gastaut syndrome: an open study". Epilepsia. 35 (5): 993–8. doi:10.1111/j.1528-1157.1994.tb02544.x. PMID 7925171. S2CID 24204172.
- ↑ Zwanzger P, Baghai TC, Schuele C, Strohle A, Padberg F, Kathmann N, Schwarz M, Moller HJ, Rupprecht R (2001). "Vigabatrin decreases cholecystokinin-tetrapeptide (CCK-4) induced panic in healthy volunteers". Neuropsychopharmacology. 25 (5): 699–703. doi:10.1016/S0893-133X(01)00266-4. PMID 11682253.
- ↑ Pearl, Phillip L; Robbins, Emily; Capp, Philip K; Gasior, Maciej; Gibson, K Michael (May 5, 2004). "Succinic Semialdehyde Dehydrogenase Deficiency". GeneReviews. Seattle, Washington: University of Washington. Archived from the original on July 28, 2020. Retrieved September 6, 2010.
- ↑ Sander JW, Hart YM (1990). "Vigabatrin and behaviour disturbance". Lancet. 335 (8680): 57. doi:10.1016/0140-6736(90)90190-G. PMID 1967367. S2CID 34456538.
- ↑ Chiaretti A, Castorina M, Tortorolo L, Piastra M, Polidori G (1994). "[Acute psychosis and vigabatrin in childhood]". La Pediatria Medica e Chirurgica : Medical and Surgical Pediatrics (in italiano). 16 (5): 489–90. PMID 7885961.
- ↑ Sander JW, Hart YM, Trimble MR, Shorvon SD (1991). "Vigabatrin and psychosis". Journal of Neurology, Neurosurgery, and Psychiatry. 54 (5): 435–9. doi:10.1136/jnnp.54.5.435. PMC 488544. PMID 1865207.
- ↑ Abdulrazzaq YM, Padmanabhan R, Bastaki SM, Ibrahim A, Bener A (2001). "Placental transfer of vigabatrin (gamma-vinyl GABA) and its effect on concentration of amino acids in the embryo of TO mice". Teratology. 63 (3): 127–33. doi:10.1002/tera.1023. PMID 11283969.
- ↑ Lombardo SA, Leanza G, Meli C, Lombardo ME, Mazzone L, Vincenti I, Cioni M (2005). "Maternal exposure to the antiepileptic drug vigabatrin affects postnatal development in the rat" (PDF). Neurological Sciences. 26 (2): 89–94. doi:10.1007/s10072-005-0441-6. hdl:2108/194069. PMID 15995825. S2CID 25257244. Archived (PDF) from the original on 2021-08-27. Retrieved 2021-07-29.
- ↑ Frisén L, Malmgren K (2003). "Characterization of vigabatrin-associated optic atrophy". Acta Ophthalmologica Scandinavica. 81 (5): 466–73. doi:10.1034/j.1600-0420.2003.00125.x. PMID 14510793.
- ↑ Buncic JR, Westall CA, Panton CM, Munn JR, MacKeen LD, Logan WJ (2004). "Characteristic retinal atrophy with secondary "inverse" optic atrophy identifies vigabatrin toxicity in children". Ophthalmology. 111 (10): 1935–42. doi:10.1016/j.ophtha.2004.03.036. PMC 3880364. PMID 15465561.
- ↑ Gaucher D; Arnault E; Husson Z; et al. (November 2012). "Taurine deficiency damages retinal neurones: cone photoreceptors and retinal ganglion cells". Amino Acids. 43 (5): 1979–1993. doi:10.1007/s00726-012-1273-3. PMC 3472058. PMID 22476345.
- ↑ "SABRIL® (vigabatrin) tablets, for oral use SABRIL® (vigabatrin) powder for oral..." Sabril.net. Retrieved 2019-05-31.
{{cite web}}: CS1 maint: url-status (link) - ↑ Sanchez-Alcaraz, Agustín; Quintana MB; Lopez E; Rodriguez I; Llopis P (2002). "Effect of vigabatrin on the pharmacokinetics of carbamazepine". Journal of Clinical Pharmacy and Therapeutics. 27 (6): 427–30. doi:10.1046/j.1365-2710.2002.00441.x. PMID 12472982. S2CID 29986581.
- ↑ Rimmer EM, Richens A (1984). "Double-blind study of gamma-vinyl GABA in patients with refractory epilepsy". Lancet. 1 (8370): 189–90. doi:10.1016/S0140-6736(84)92112-3. PMID 6141335. S2CID 54336689.
- ↑ Rimmer EM, Richens A (1989). "Interaction between vigabatrin and phenytoin". British Journal of Clinical Pharmacology. 27 (Suppl 1): 27S–33S. doi:10.1111/j.1365-2125.1989.tb03458.x. PMC 1379676. PMID 2757906.
- ↑ Storici Paola; De Biase D; Bossa F; Bruno S; Mozzarelli A; Peneff C; Silverman R; Schirmer T. (2003). "Structures of γ-Aminobutyric Acid (GABA) Aminotransferase, a Pyridoxal 5'-Phosphate, and [2Fe-2S] Cluster-containing Enzyme, Complexed with γ-Ethynyl-GABA and with the Antiepilepsy Drug Vigabatrin". The Journal of Biological Chemistry. 279 (1): 363–73. doi:10.1074/jbc.M305884200. PMID 14534310.
- ↑ Rogawski MA, Löscher W (2004). "The neurobiology of antiepileptic drugs". Nat Rev Neurosci. 5 (7): 553–564. doi:10.1038/nrn1430. PMID 15208697. S2CID 2201038. Archived from the original on 2020-12-16. Retrieved 2021-07-29.
- ↑ Sheean, G.; Schramm T; Anderson DS; Eadie MJ. (1992). "Vigabatrin--plasma enantiomer concentrations and clinical effects". Clinical and Experimental Neurology. 29: 107–16. PMID 1343855.
- ↑ 27.0 27.1 Gram L, Larsson OM, Johnsen A, Schousboe A (1989). "Experimental studies of the influence of vigabatrin on the GABA system". British Journal of Clinical Pharmacology. 27 (Suppl 1): 13S–17S. doi:10.1111/j.1365-2125.1989.tb03455.x. PMC 1379673. PMID 2757904.
- ↑ Browne TR (1998). "Pharmacokinetics of antiepileptic drugs". Neurology. 51 (5 suppl 4): S2–7. doi:10.1212/wnl.51.5_suppl_4.s2. PMID 9818917. S2CID 39231047.
- ↑ Lindberger M, Luhr O, Johannessen SI, Larsson S, Tomson T (2003). "Serum concentrations and effects of gabapentin and vigabatrin: observations from a dose titration study". Therapeutic Drug Monitoring. 25 (4): 457–62. doi:10.1097/00007691-200308000-00007. PMID 12883229. S2CID 35834401.
- ↑ Ben-Menachem E. (2011). "Mechanism of Action of vigabatrin: correcting misperceptions". Acta Neurologica Scandinavica. 124 (192): 5–15. doi:10.1111/j.1600-0404.2011.01596.x. PMID 22061176. S2CID 25347559.
- ↑ "drugs.com Vigabatrin Drug Information". Archived from the original on 2020-07-28. Retrieved 2021-07-29.
- ↑ Treatments for Epilepsy - Vigabatrin Norfolk and Waveney Mental Health Partnership NHS Trust
- ↑ "FDA approves first generic version of Sabril to help treat seizures in adults and pediatric patients with epilepsy". U.S. Food and Drug Administration (FDA) (Press release). 2019-09-11. Archived from the original on 2021-01-27. Retrieved 2021-07-29.
External links
| External sites: | |
|---|---|
| Identifiers: |
|
- Pages using duplicate arguments in template calls
- CS1 maint: date format
- Webarchive template wayback links
- CS1 maint: PMC embargo expired
- CS1 italiano-language sources (it)
- CS1 maint: url-status
- Drugs with non-standard legal status
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without UNII source
- Drugs missing an ATC code
- Drugboxes which contain changes to watched fields
- Alkene derivatives
- Amines
- Anticonvulsants
- Carboxylic acids
- GABA transaminase inhibitors
- GABA analogues
- RTT