Oteseconazole
 | |
| Names | |
|---|---|
| Trade names | Vivjoa |
| Other names | VT-1161 |
| |
| Clinical data | |
| Drug class | Antifungal |
| Main uses | Recurrent vaginal yeast infections[1] |
| Side effects | Headache, nausea[1] |
| Pregnancy category |
|
| Routes of use | By mouth |
| External links | |
| AHFS/Drugs.com | Monograph |
| Legal | |
| License data | |
| Legal status | |
| Chemical and physical data | |
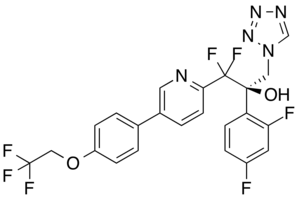
| Formula | C23H16F7N5O2 |
| Molar mass | 527.403 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Oteseconazole, sold under the brand name Vivjoa, is a medication used to decrease the frequency of recurrent vaginal yeast infections.[1] It is taken by mouth.[1] It may be used with fluconazole.[1]
Commons side effects include headache and nausea.[1] Use within two years of pregnancy may harm the baby and thus it should not be used in women who could become pregnant.[1] Use is not recommended in those with significant kidney or liver problems.[1] It is a 14α-demethylase (CYP51) inhibitor.[4]
Oteseconazole was approved for medical use in the United States in 2022.[1] It is not approved in Europe or the United Kingdom as of 2022.[4] In the United States a course of treatment costs about 2,900 USD as of 2022.[5]
Medical use
Dosage
It is initially taken at a dose of 600 mg followed by 450 mg the next day.[1] Starting on day 14 it is than taken at a dose of 150 mg once per week for 11 weeks.[1]
Society and culture
It was developed by Mycovia Pharmaceuticals.[6]
Names
Oteseconazole is the international nonproprietary name (INN).[7]
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 "DailyMed - VIVJOA- oteseconazole capsule". dailymed.nlm.nih.gov. Archived from the original on 13 August 2022. Retrieved 12 December 2022.
- ↑ "Oteseconazole (Vivjoa) Use During Pregnancy". Drugs.com. Retrieved 12 December 2022.
- ↑ https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/215888s000lbl.pdf Archived 2022-05-23 at the Wayback Machine[bare URL PDF]
- ↑ 4.0 4.1 "Oteseconazole". SPS - Specialist Pharmacy Service. 3 April 2021. Archived from the original on 8 August 2022. Retrieved 12 December 2022.
- ↑ "Vivjoa Prices, Coupons, Copay & Patient Assistance". Drugs.com. Retrieved 12 December 2022.
- ↑ "FDA Approves Mycovia Pharmaceuticals' VIVJOA (oteseconazole), the First and Only FDA-Approved Medication for Recurrent Vulvovaginal Candidiasis (Chronic Yeast Infection)" (Press release). Mycovia Pharmaceuticals. 28 April 2022. Archived from the original on 28 April 2022. Retrieved 28 April 2022 – via Business Wire.
- ↑ World Health Organization (2016). "International nonproprietary names for pharmaceutical substances (INN): recommended INN: list 76". WHO Drug Information. 30 (3). hdl:10665/331020.
External links
| External sites: | |
|---|---|
| Identifiers: |
|
- Sobel JD, Nyirjesy P (December 2021). "Oteseconazole: an advance in treatment of recurrent vulvovaginal candidiasis". Future Microbiology. 16 (18): 1453–1461. doi:10.2217/fmb-2021-0173. PMID 34783586.
- Clinical trial number NCT03562156 for "A Study of Oral Oteseconazole for the Treatment of Patients With Recurrent Vaginal Candidiasis (Yeast Infection) (VIOLET)" at ClinicalTrials.gov
- Clinical trial number NCT03561701 for "A Study of Oral Oteseconazole (VT-1161) for the Treatment of Patients With Recurrent Vaginal Candidiasis (Yeast Infection) (VIOLET)" at ClinicalTrials.gov
- Clinical trial number NCT03840616 for "Study of Oral Oteseconazole (VT-1161) for Acute Yeast Infections in Patients With Recurrent Yeast Infections (ultraVIOLET)" at ClinicalTrials.gov
- Pages using duplicate arguments in template calls
- Webarchive template wayback links
- All articles with bare URLs for citations
- Articles with bare URLs for citations from May 2022
- Articles with invalid date parameter in template
- Articles with PDF format bare URLs for citations
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without UNII source
- Drugs missing an ATC code
- Articles containing unverified chemical infoboxes
- Trifluoromethyl compounds
- Fluoroarenes
- Pyridines
- Tetrazoles
- Tertiary alcohols
- RTT