Lifitegrast
 | |
| Names | |
|---|---|
| Pronunciation | Xiidra /ˈzaɪdrə/[1] |
| Trade names | Xiidra |
| Other names | SAR-1118 |
| |
| Clinical data | |
| Main uses | Dry eyes[2] |
| Side effects | Blurry vision, red eyes, headache, change in taste, itchiness[2] |
| Pregnancy category |
|
| Routes of use | Eye drops |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a616039 |
| Legal | |
| Legal status | |
| Chemical and physical data | |
| Formula | C29H24Cl2N2O7S |
| Molar mass | 615.48 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Lifitegrast, sold under the brand name Xiidra, is a medication used to treat dry eyes.[2] It is unclear if its benefits are greater than its harms.[3][4] It is used as eye drops.[2]
Common side effects include blurry vision, red eyes, headache, change in taste, and itchiness.[2] Safety in pregnancy is not clear.[5] It reduces inflammation by inhibiting T cells.[3]
Lifitegrast was approved for medical use in the United States in 2016.[2] It is not approved in either the United Kingdom or Europe.[3][4] In the United States it costs about 580 USD per month as of 2021.[6]
Medical uses
Dosage
It is used as once drop twice per day of 5% solution.[2] Lifitegrast is supplied as an eye drop.
Side effects
Common side effects are the following:[7]
- Eye irritation
- Discomfort
- Blurred vision
- Dysgeusia ( distortion of the sense of taste)
Pharmacology
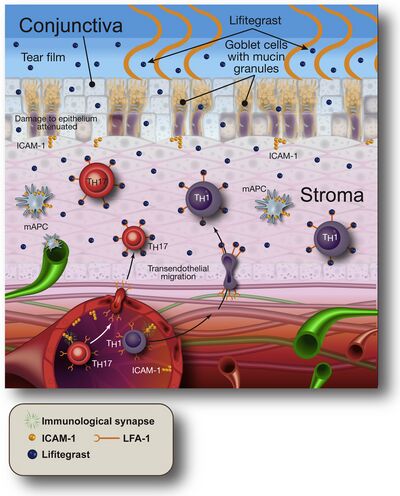
Mechanism of action
Lifitegrast inhibits an integrin, lymphocyte function-associated antigen 1 (LFA-1), from binding to intercellular adhesion molecule 1 (ICAM-1). This mechanism down-regulates inflammation mediated by T lymphocytes.[9][10]
History
Lifitegrast was initially designed and developed by SARcode Bioscience[11] which was acquired by Shire in 2013,[12] which submitted a new drug application to the US Food and Drug Administration (FDA) in March 2015.
The FDA granted Shire a priority review a month later, and requested additional clinical data, which were supplied in January 2016; approval was granted on 11 July 2016.[13][14]
Lifitegrast was approved by Health Canada in January 2018, and available in Canadian pharmacies as of March 2018.
Shire was acquired by Takeda Pharmaceutical Company in late 2018.[15]
In 2019 Novartis reached an agreement to purchase the assets associated with Lifitegrast. Novartis will pay Takeda an upfront payment of $3.4 billion, while the latter drugmaker is eligible for milestone payments of as much as $1.9 billion. Novartis noted that the drug amassed approximately $400 million in revenue in 2018.[16]
Society and culture
Cost
The medication is sold in the U.S. at a cost of $587 (USD) for 60 solution(s) ophthalmic solution 5% [17]
-
Lifitegrast costs (US)
-
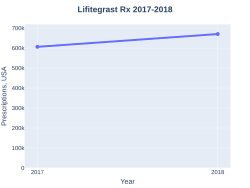
Lifitegrast prescriptions (US)
References
- ↑ "Patient information: Xiidra® (ZYE-druh) (lifitegrast ophthalmic solution) 5% for topical ophthalmic use" (PDF). Novartis. June 2020. Archived (PDF) from the original on 2021-02-13. Retrieved 2021-02-05.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 "Lifitegrast Monograph for Professionals". Drugs.com. Archived from the original on 5 March 2021. Retrieved 22 November 2021.
- ↑ 3.0 3.1 3.2 "Lifitegrast". SPS - Specialist Pharmacy Service. 8 February 2016. Archived from the original on 11 December 2021. Retrieved 23 November 2021.
- ↑ 4.0 4.1 "Xiidra: Withdrawal of the marketing authorisation application". Archived from the original on 14 November 2021. Retrieved 23 November 2021.
- ↑ "Lifitegrast ophthalmic (Xiidra) Use During Pregnancy". Drugs.com. Archived from the original on 28 November 2020. Retrieved 23 November 2021.
- ↑ "Lifitegrast Prices, Coupons & Savings Tips - GoodRx". GoodRx. Archived from the original on 28 October 2016. Retrieved 23 November 2021.
- ↑ Drugs.com: Patient information for xiidra.
- ↑ Perez, Victor L.; Pflugfelder, Stephen C.; Zhang, Steven; Shojaei, Amir; Haque, Reza (1 April 2016). "Lifitegrast, a Novel Integrin Antagonist for Treatment of Dry Eye Disease". The Ocular Surface. 14 (2): 207–215. doi:10.1016/j.jtos.2016.01.001. ISSN 1542-0124.
- ↑ Tauber J, Karpecki P, Latkany R, Luchs J, Martel J, Sall K, et al. (December 2015). "Lifitegrast Ophthalmic Solution 5.0% versus Placebo for Treatment of Dry Eye Disease: Results of the Randomized Phase III OPUS-2 Study". Ophthalmology. 122 (12): 2423–31. doi:10.1016/j.ophtha.2015.08.001. PMID 26365210.
- ↑ Murphy CJ, Bentley E, Miller PE, McIntyre K, Leatherberry G, Dubielzig R, et al. (May 2011). "The pharmacologic assessment of a novel lymphocyte function-associated antigen-1 antagonist (SAR 1118) for the treatment of keratoconjunctivitis sicca in dogs". Investigative Ophthalmology & Visual Science. 52 (6): 3174–80. doi:10.1167/iovs.09-5078. PMID 21330663.
- ↑ Semba CP, Gadek TR (2016). "Development of lifitegrast: a novel T-cell inhibitor for the treatment of dry eye disease". Clinical Ophthalmology. 10: 1083–94. doi:10.2147/OPTH.S110557. PMC 4910612. PMID 27354762.
- ↑ "Shire To Acquire Sarcode Bioscience, Expands Presence In Ophthalmology". 25 March 2013. Archived from the original on 26 November 2017. Retrieved 16 September 2016.
- ↑ "FDA Approves Shire's Xiidra". 11 July 2016. Archived from the original on 17 August 2016. Retrieved 13 July 2016.
- ↑ Drugs.com: Xiidra (lifitegrast) FDA Approval History Archived 2016-09-14 at the Wayback Machine
- ↑ "Takeda Completes Acquisition of Shire, Becoming a Global, Values-based, R&D-Driven Biopharmaceutical Leader". Takeda. January 8, 2019. Archived from the original on June 24, 2021. Retrieved May 13, 2019.
- ↑ "Novartis to acquire Xiidra, expanding front-of-eye portfolio and strengthening leadership in eye care". Novartis (Press release). May 9, 2019. Archived from the original on May 13, 2019. Retrieved May 13, 2019.
- ↑ "Xiidra Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 17 January 2021. Retrieved 3 April 2021.
External links
| Identifiers: |
|
|---|
- Pages using duplicate arguments in template calls
- Webarchive template wayback links
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without UNII source
- Drugs missing an ATC code
- Articles containing unverified chemical infoboxes
- Ophthalmology drugs
- Amino acids
- Benzosulfones
- Takeda Pharmaceutical Company brands
- Novartis brands
- Benzamides
- RTT