Irbesartan
 | |
| Names | |
|---|---|
| Pronunciation | /ɜːrbəˈsɑːrtən/ |
| Trade names | Avapro, others |
| |
| Clinical data | |
| Drug class | Angiotensin II receptor antagonist (ARB)[1] |
| Main uses | High blood pressure, heart failure, diabetic kidney disease[1] |
| Side effects | Dizziness, diarrhea, feeling tired, muscle pain, heartburn[1][2] |
| Pregnancy category | |
| Routes of use | By mouth |
| Defined daily dose | 150 mg[4] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a698009 |
| Legal | |
| License data |
|
| Legal status | |
| Pharmacokinetics | |
| Bioavailability | 60% to 80% |
| Protein binding | ~90% |
| Metabolism | Liver (CYP2C9) |
| Elimination half-life | 11 h to 15 h |
| Excretion | Kidney 20%, feces 65% |
| Chemical and physical data | |
| Formula | C25H28N6O |
| Molar mass | 428.540 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Irbesartan, sold under the brand name Avapro among others, is a medication used to treat high blood pressure, heart failure, and diabetic kidney disease.[1] It is a reasonable initial treatment for high blood pressure.[1] It is taken by mouth.[1] Versions are available as the combination irbesartan/hydrochlorothiazide.[1][5]
Common side effects include dizziness, diarrhea, feeling tired, muscle pain, and heartburn.[1][2] Serious side effects may include kidney problems, low blood pressure, and angioedema.[1] Use in pregnancy may harm the baby and use when breastfeeding is not recommended.[6] It is an angiotensin II receptor antagonist and works by blocking the effects of angiotensin II.[1]
Irbesartan was patented in 1990, and approved for medical use in 1997.[7] It is available as a generic medication.[2] A month supply in the United Kingdom costs the NHS less than two pounds as of 2019.[2] In the United States the wholesale cost of this amount is about six dollars.[8] In 2017, it was the 220th most commonly prescribed medication in the United States, with more than two million prescriptions.[9][10]
Medical uses
As with all angiotensin II receptor antagonists, irbesartan is used for the treatment of high blood pressure. It may also delay progression of diabetic nephropathy and the reduction of kidney disease progression in type 2 diabetes,[11] hypertension and microalbuminuria (>30 mg/24 h) or proteinuria (>900 mg/24 h).[12]
Irbesartan is also available in a combination formulation with a low-dose thiazide diuretic, invariably hydrochlorothiazide, to achieve an additive antihypertensive effect.[5][13] Irbesartan/hydrochlorothiazide combination preparations are marketed under various brand names.[14]
Dosage
The defined daily dose is 150 mg by mouth.[4]
Society and culture
It was developed by Sanofi Research (part of Sanofi-Aventis). It is jointly marketed by Sanofi-Aventis and Bristol-Myers Squibb under the brand names Aprovel, Karvea, and Avapro.[5][3]
Cost
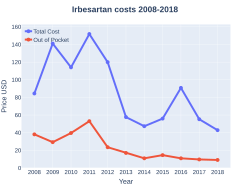
A month supply in the United Kingdom costs the NHS less than two pounds as of 2019.[2] In the United States the wholesale cost of this amount is about six dollars.[8]In 2017, it was the 220th most commonly prescribed medication in the United States, with more than two million prescriptions.[9][10]
-
Irbesartan costs (US)
-
Irbesartan prescriptions (US)
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 1.9 "Irbesartan Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Archived from the original on 30 November 2019. Retrieved 3 March 2019.
- ↑ 2.0 2.1 2.2 2.3 2.4 British national formulary : BNF 76 (76 ed.). Pharmaceutical Press. 2018. p. 175. ISBN 9780857113382.
- ↑ 3.0 3.1 3.2 "Irbesartan (Avapro) Use During Pregnancy". Drugs.com. 16 August 2018. Archived from the original on 2 May 2019. Retrieved 19 March 2020.
- ↑ 4.0 4.1 "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 21 January 2021. Retrieved 7 September 2020.
- ↑ 5.0 5.1 5.2 "Avalide- irbesartan and hydrochlorothiazide tablet, film coated". DailyMed. 31 July 2018. Archived from the original on 15 February 2018. Retrieved 19 March 2020.
- ↑ "Irbesartan Pregnancy and Breastfeeding Warnings". Drugs.com. Archived from the original on 2 May 2019. Retrieved 3 March 2019.
- ↑ Fischer, Jnos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 470. ISBN 9783527607495. Archived from the original on 2021-08-28. Retrieved 2020-06-30.
- ↑ 8.0 8.1 "NADAC as of 2019-02-27". Centers for Medicare and Medicaid Services. Archived from the original on 2019-03-06. Retrieved 3 March 2019.
- ↑ 9.0 9.1 "The Top 300 of 2020". ClinCalc. Archived from the original on 12 February 2021. Retrieved 11 April 2020.
- ↑ 10.0 10.1 "Irbesartan - Drug Usage Statistics". ClinCalc. Archived from the original on 11 April 2020. Retrieved 11 April 2020.
- ↑ Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, Ritz E, Atkins RC, Rohde R, Raz I, Collaborative Study Group (2001). "Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes". N Engl J Med. 345 (12): 851–60. doi:10.1056/NEJMoa011303. hdl:2445/122787. PMID 11565517.
- ↑ Rossi S, editor. Australian Medicines Handbook 2006. Adelaide: Australian Medicines Handbook; 2006. ISBN 0-9757919-2-3
- ↑ "Irbesartan and Hydrochlorothiazide (Professional Patient Advice)". Drugs.com. 5 June 2019. Archived from the original on 20 March 2020. Retrieved 19 March 2020.
- ↑ "Irbesartan and hydrochlorothiazide Advanced Patient Information". Drugs.com. 24 December 2019. Archived from the original on 20 March 2020. Retrieved 19 March 2020.
External links
| External sites: | |
|---|---|
| Identifiers: |
|
- "Hydrochlorothiazide mixture with irbesartan". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 2020-06-12. Retrieved 2020-03-20.
- Pages using duplicate arguments in template calls
- Drugs with non-standard legal status
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without UNII source
- Drugs missing an ATC code
- Drug has EMA link
- Drugboxes which contain changes to watched fields
- Angiotensin II receptor antagonists
- Sanofi
- Bristol-Myers Squibb
- Tetrazoles
- Biphenyls
- Lactams
- Spiro compounds
- Nitrogen heterocycles
- RTT