Yellow fever
| Yellow fever | |
|---|---|
| Other names: Yellow jack, yellow plague,[1] bronze john[2] | |
 | |
| A TEM micrograph of yellow fever virus (234,000× magnification) | |
| Specialty | Infectious disease |
| Symptoms | Fever, chills, muscle pain, headache, yellow skin[3] |
| Complications | Liver failure, bleeding[3] |
| Usual onset | 3 – 6 days post exposure[3] |
| Duration | 3 – 4 days[3] |
| Causes | Yellow fever virus spread by mosquitoes[3] |
| Diagnostic method | Blood test[4] |
| Prevention | Yellow fever vaccine[3] |
| Treatment | Supportive care[3] |
| Frequency | ~127,000 severe cases (2013)[3] |
| Deaths | 5,100 (2015)[5] |
| |
Yellow fever is a viral disease of typically short duration.[3] In most cases, symptoms include fever, chills, loss of appetite, nausea, muscle pains particularly in the back, and headaches.[3] Symptoms typically improve within five days.[3] In about 15% of people, within a day of improving the fever comes back, abdominal pain occurs, and liver damage begins causing yellow skin.[3][6] If this occurs, the risk of bleeding and kidney problems is increased.[3]
The disease is caused by yellow fever virus and is spread by the bite of an infected female mosquito.[3] It infects only humans, other primates, and several types of mosquitoes.[3] In cities, it is spread primarily by Aedes aegypti, a type of mosquito found throughout the tropics and subtropics.[3] The virus is an RNA virus of the genus Flavivirus.[7] The disease may be difficult to tell apart from other illnesses, especially in the early stages.[3] To confirm a suspected case, blood-sample testing with polymerase chain reaction is required.[4]
A safe and effective vaccine against yellow fever exists, and some countries require vaccinations for travelers.[3] Other efforts to prevent infection include reducing the population of the transmitting mosquitoes.[3] In areas where yellow fever is common, early diagnosis of cases and immunization of large parts of the population are important to prevent outbreaks.[3] Once infected, management is symptomatic with no specific measures effective against the virus.[3] Death occurs in up to half of those who get severe disease.[3][8]
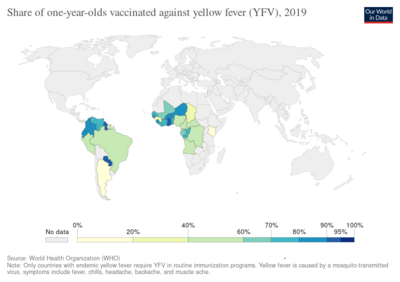
In 2013, yellow fever resulted in about 127,000 severe infections and 45,000 deaths,[3] with nearly 90 percent of these occurring in African nations.[4] Nearly a billion people live in an area of the world where the disease is common.[3] It is common in tropical areas of the continents of South America and Africa, but not in Asia.[3][9] Since the 1980s, the number of cases of yellow fever has been increasing.[3][10] This is believed to be due to fewer people being immune, more people living in cities, people moving frequently, and changing climate increasing the habitat for mosquitoes.[3] The disease originated in Africa and spread to South America with the slave trade in the 17th century.[1] Since the 17th century, several major outbreaks of the disease have occurred in the Americas, Africa, and Europe.[1] In the 18th and 19th centuries, yellow fever was seen as one of the most dangerous infectious diseases.[1] In 1927, yellow fever virus was the first human virus isolated.[7][11]
Signs and symptoms
Yellow fever begins after an incubation period of three to six days.[12] Most cases only cause a mild infection with fever, headache, chills, back pain, fatigue, loss of appetite, muscle pain, nausea, and vomiting, in these cases, the infection lasts three to four days.[13]
In 15% of cases, though, people enter a second, toxic phase of the disease with recurring fever, this time accompanied by jaundice due to liver damage, as well as abdominal pain.[14] Bleeding in the mouth, nose, the eyes, and the gastrointestinal tract cause vomit containing blood, hence the Spanish name for yellow fever, vómito negro ("black vomit").[15] There may also be kidney failure, hiccups, and delirium.[16][17]
Among those who develop jaundice, the fatality rate is 20 to 50%, while the overall fatality rate is about 3 to 7.5%.[18] Severe cases may have a mortality greater than 50%.[19]
Surviving the infection provides lifelong immunity,[20] and normally no permanent organ damage results.[21]
Cause
| Yellow fever virus | |
|---|---|
| Virus classification | |
| (unranked): | Virus |
| Realm: | Riboviria |
| Kingdom: | Orthornavirae |
| Phylum: | Kitrinoviricota |
| Class: | Flasuviricetes |
| Order: | Amarillovirales |
| Family: | Flaviviridae |
| Genus: | Flavivirus |
| Species: | Yellow fever virus
|
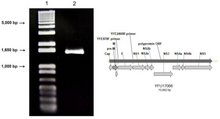
Yellow fever is caused by yellow fever virus, an enveloped RNA virus 40–50 nm in width, the type species and namesake of the family Flaviviridae.[7] It was the first illness shown to be transmissible by filtered human serum and transmitted by mosquitoes, by Walter Reed around 1900.[22] The positive-sense, single-stranded RNA is around 11,000 nucleotides long and has a single open reading frame encoding a polyprotein. Host proteases cut this polyprotein into three structural (C, prM, E) and seven nonstructural proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B, NS5); the enumeration corresponds to the arrangement of the protein coding genes in the genome.[23] Minimal yellow fever virus (YFV) 3'UTR region is required for stalling of the host 5'-3' exonuclease XRN1. The UTR contains PKS3 pseudoknot structure, which serves as a molecular signal to stall the exonuclease and is the only viral requirement for subgenomic flavivirus RNA (sfRNA) production. The sfRNAs are a result of incomplete degradation of the viral genome by the exonuclease and are important for viral pathogenicity.[24] Yellow fever belongs to the group of hemorrhagic fevers.[25]
The viruses infect, amongst others, monocytes, macrophages, Schwann cells, and dendritic cells. They attach to the cell surfaces via specific receptors and are taken up by an endosomal vesicle. Inside the endosome, the decreased pH induces the fusion of the endosomal membrane with the virus envelope. The capsid enters the cytosol, decays, and releases the genome. Receptor binding, as well as membrane fusion, are catalyzed by the protein E, which changes its conformation at low pH, causing a rearrangement of the 90 homodimers to 60 homotrimers.[23][26]
After entering the host cell, the viral genome is replicated in the rough endoplasmic reticulum (ER) and in the so-called vesicle packets. At first, an immature form of the virus particle is produced inside the ER, whose M-protein is not yet cleaved to its mature form, so is denoted as precursor M (prM) and forms a complex with protein E. The immature particles are processed in the Golgi apparatus by the host protein furin, which cleaves prM to M. This releases E from the complex, which can now take its place in the mature, infectious virion.[23]
Transmission


Yellow fever virus is mainly transmitted through the bite of the yellow fever mosquito Aedes aegypti, but other mostly Aedes mosquitoes such as the tiger mosquito (Aedes albopictus) can also serve as a vector for this virus. Like other arboviruses, which are transmitted by mosquitoes, yellow fever virus is taken up by a female mosquito when it ingests the blood of an infected human or another primate. Viruses reach the stomach of the mosquito, and if the virus concentration is high enough, the virions can infect epithelial cells and replicate there. From there, they reach the haemocoel (the blood system of mosquitoes) and from there the salivary glands. When the mosquito next sucks blood, it injects its saliva into the wound, and the virus reaches the bloodstream of the bitten person. Transovarial and transstadial transmission of yellow fever virus within A. aegypti, that is, the transmission from a female mosquito to her eggs and then larvae, are indicated. This infection of vectors without a previous blood meal seems to play a role in single, sudden breakouts of the disease.[27]
Three epidemiologically different infectious cycles occur[10] in which the virus is transmitted from mosquitoes to humans or other primates.[28] In the "urban cycle", only the yellow fever mosquito A. aegypti is involved. It is well adapted to urban areas, and can also transmit other diseases, including Zika fever, dengue fever, and chikungunya. The urban cycle is responsible for the major outbreaks of yellow fever that occur in Africa.[29][30]
Besides the urban cycle, both in Africa and South America, a sylvatic cycle (forest or jungle cycle) is present, where Aedes africanus (in Africa) or mosquitoes of the genus Haemagogus and Sabethes (in South America) serve as vectors. In the jungle, the mosquitoes infect mainly nonhuman primates; the disease is mostly asymptomatic in African primates. In South America, the sylvatic cycle is currently the only way humans can become infected, which explains the low incidence of yellow fever cases on the continent. People who become infected in the jungle can carry the virus to urban areas, where A. aegypti acts as a vector. Because of this sylvatic cycle, yellow fever cannot be eradicated except by eradicating the mosquitoes that serve as vectors.[10]
In Africa, a third infectious cycle known as "savannah cycle" or intermediate cycle, occurs between the jungle and urban cycles. Different mosquitoes of the genus Aedes are involved. In recent years, this has been the most common form of transmission of yellow fever in Africa.[31]
Concern exists about yellow fever spreading to southeast Asia, where its vector A. aegypti already occurs.[32]
Pathogenesis

After transmission from a mosquito, the viruses replicate in the lymph nodes and infect dendritic cells in particular. From there, they reach the liver and infect hepatocytes (probably indirectly via Kupffer cells), which leads to eosinophilic degradation of these cells and to the release of cytokines. Apoptotic masses known as Councilman bodies appear in the cytoplasm of hepatocytes.[34][35]
Fatality may occur when cytokine storm, shock, and multiple organ failure follow.[18]
Diagnosis
Yellow fever is most frequently a clinical diagnosis, based on symptomatology and travel history. Mild cases of the disease can only be confirmed virologically. Since mild cases of yellow fever can also contribute significantly to regional outbreaks, every suspected case of yellow fever involving fever, pain, nausea, and vomiting after having been in an affected area, is treated seriously.[36][25][37]
If yellow fever is suspected, the virus cannot be confirmed until 6–10 days following the illness. A direct confirmation can be obtained by reverse transcription polymerase chain reaction, where the genome of the virus is amplified.[4] Another direct approach is the isolation of the virus and its growth in cell culture.[37]
Serologically, an enzyme-linked immunosorbent assay during the acute phase of the disease using specific IgM against yellow fever or an increase in specific IgG titer (compared to an earlier sample) can confirm yellow fever. Together with clinical symptoms, the detection of IgM or a four-fold increase in IgG titer is considered sufficient indication for yellow fever. As these tests can cross-react with other flaviviruses, such as dengue virus, these indirect methods cannot conclusively prove yellow fever infection. Liver biopsy can verify inflammation and necrosis of hepatocytes and detect viral antigens. Because of the bleeding tendency of yellow fever patients, a biopsy is only advisable post mortem to confirm the cause of death.[36][25][38]
Differential diagnosis
In a differential diagnosis, infections with yellow fever must be distinguished from other feverish illnesses such as malaria. Other viral hemorrhagic fevers, such as Ebola virus, Lassa virus, Marburg virus, and Junin virus, must be excluded as the cause.[25][39]
Prevention
Personal prevention of yellow fever includes vaccination and avoidance of mosquito bites in areas where yellow fever is endemic. Institutional measures for prevention of yellow fever include vaccination programmes and measures to control mosquitoes. Programmes for distribution of mosquito nets for use in homes produce reductions in cases of both malaria and yellow fever. Use of EPA-registered insect repellent is recommended when outdoors. Exposure for even a short time is enough for a potential mosquito bite. Long-sleeved clothing, long pants, and socks are useful for prevention. The application of larvicides to water-storage containers can help eliminate potential mosquito breeding sites. EPA-registered insecticide spray decreases the transmission of yellow fever.[40] As well as the following:
- Use of insect repellent when outdoors such as those containing DEET, picaridin, para-menthane-diol or oil of lemon eucalyptus.[40][41]
- Wear long sleeves, long pants, and socks when outdoors. Mosquitoes may bite through thin clothing, so spraying clothes with repellent containing permethrin or another EPA-registered repellent gives extra protection.[40][41]
- The peak biting times for many mosquito species are dusk to dawn, however, A. aegypti, one of the mosquitoes that transmits yellow fever virus, feeds during the daytime; therefore, staying in accommodations with screened or air-conditioned rooms, during peak biting times, also reduces the risk of mosquito bites.[40][8]
Vaccination
Vaccination is recommended for those traveling to affected areas, because non-native people tend to develop more severe illness when infected. Protection begins by the 10th day after vaccine administration in 95% of people,[42] and had been reported to last for at least 10 years. The World Health Organization (WHO) now states that a single dose of vaccine is sufficient to confer lifelong immunity against yellow fever disease."[43] The attenuated live vaccine stem 17D was developed in 1937 by Max Theiler.[42] The WHO recommends routine vaccination for people living in affected areas between the 9th and 12th month after birth.[4]
Up to one in four people experience fever, aches, and local soreness and redness at the site of injection.[44] In rare cases (less than one in 200,000 to 300,000),[42] the vaccination can cause yellow fever vaccine-associated viscerotropic disease, which is fatal in 60% of cases. It is probably due to the genetic morphology of the immune system. Another possible side effect is an infection of the nervous system, which occurs in one in 200,000 to 300,000 cases, causing yellow fever vaccine-associated neurotropic disease, which can lead to meningoencephalitis and is fatal in less than 5%[42] of cases.[4][18]
The Yellow Fever Initiative, launched by the WHO in 2006, vaccinated more than 105 million people in 14 countries in West Africa.[45] No outbreaks were reported during 2015. The campaign was supported by the GAVI Alliance, and governmental organizations in Europe and Africa. According to the WHO, mass vaccination cannot eliminate yellow fever because of the vast number of infected mosquitoes in urban areas of the target countries, but it will significantly reduce the number of people infected.[46]
Demand for yellow fever vaccine has continued to increase due to the growing number of countries implementing yellow fever vaccination as part of their routine immunization programmes.[47] Recent upsurges in yellow fever outbreaks in Angola (2015), the Democratic Republic of Congo (2016), Uganda (2016), and more recently in Nigeria and Brazil in 2017 have further increased demand, while straining global vaccine supply.[47][48] Therefore, to vaccinate susceptible populations in preventive mass immunization campaigns during outbreaks, fractional dosing of the vaccine is being considered as a dose-sparing strategy to maximize limited vaccine supplies.[47] Fractional dose yellow fever vaccination refers to administration of a reduced volume of vaccine dose, which has been reconstituted as per manufacturer recommendations.[47][49] The first practical use of fractional dose yellow fever vaccination was in response to a large yellow fever outbreak in the Democratic Republic of the Congo in mid-2016.[47]
In March 2017, the WHO launched a vaccination campaign in Brazil with 3.5 million doses from an emergency stockpile.[50] In March 2017 the WHO recommended vaccination for travellers to certain parts of Brazil.[51] In March 2018, Brazil shifted its policy and announced it planned to vaccinate all 77.5 million currently unvaccinated citizens by April 2019.[52]
Compulsory vaccination

Some countries in Asia are considered to be potentially in danger of yellow fever epidemics, as both mosquitoes with the capability to transmit yellow fever as well as susceptible monkeys are present. The disease does not yet occur in Asia. To prevent introduction of the virus, some countries demand previous vaccination of foreign visitors who have passed through yellow fever areas. Vaccination has to be proved by a vaccination certificate, which is valid 10 days after the vaccination and lasts for 10 years. Although the WHO on 17 May 2013 advised that subsequent booster vaccinations are unnecessary, an older (than 10 years) certificate may not be acceptable at all border posts in all affected countries. A list of the countries that require yellow fever vaccination is published by the WHO.[53] If the vaccination cannot be given for some reason, dispensation may be possible. In this case, an exemption certificate issued by a WHO-approved vaccination center is required. Although 32 of 44 countries where yellow fever occurs endemically do have vaccination programmes, in many of these countries, less than 50% of their population is vaccinated.[4]
Vector control

Control of the yellow fever mosquito A. aegypti is of major importance, especially because the same mosquito can also transmit dengue fever and chikungunya disease. A. aegypti breeds preferentially in water, for example, in installations by inhabitants of areas with precarious drinking water supplies, or in domestic refuse, especially tires, cans, and plastic bottles. These conditions are common in urban areas in developing countries.[54][55][56]
Two main strategies are employed to reduce A. aegypti populations. One approach is to kill the developing larvae. Measures are taken to reduce the water accumulations in which the larvae develop. Larvicides are used, along with larvae-eating fish and copepods, which reduce the number of larvae. For many years, copepods of the genus Mesocyclops have been used in Vietnam for preventing dengue fever. This eradicated the mosquito vector in several areas. Similar efforts may prove effective against yellow fever. Pyriproxyfen is recommended as a chemical larvicide, mainly because it is safe for humans and effective in small doses.[4]
The second strategy is to reduce populations of the adult yellow fever mosquito. Lethal ovitraps can reduce Aedes populations, using lesser amounts of pesticide because it targets the pest directly. Curtains and lids of water tanks can be sprayed with insecticides, but application inside houses is not recommended by the WHO. Insecticide-treated mosquito nets are effective, just as they are against the Anopheles mosquito that carries malaria.[4]
Treatment
As with other Flavivirus infections, no cure is known for yellow fever. Hospitalization is advisable and intensive care may be necessary because of rapid deterioration in some cases. Certain acute treatment methods lack efficacy: passive immunization after the emergence of symptoms is probably without effect; ribavirin and other antiviral drugs, as well as treatment with interferons, are ineffective in yellow fever patients.[18] Symptomatic treatment includes rehydration and pain relief with drugs such as paracetamol. Acetylsalicylic acid (aspirin) should not be given because of its anticoagulant effect, which can be devastating in the case of internal bleeding that may occur with yellow fever.[25][57][58]
Epidemiology
Yellow fever is common in tropical and subtropical areas of South America and Africa. The U.S. Centers for Disease Control and Prevention estimates that 200,000 cases of disease and 30,000 deaths a year occur, but the number of officially reported cases is far lower.[59][60][61]
Africa

An estimated 90% of yellow fever infections occur on the African continent.[4] In 2016, a large outbreak originated in Angola and spread to neighboring countries before being contained by a massive vaccination campaign. In March and April 2016, 11 imported cases of the Angola genotype in unvaccinated Chinese nationals were reported in China, the first appearance of the disease in Asia in recorded history.[62][63]
Phylogenetic analysis has identified seven genotypes of yellow fever viruses, and they are assumed to be differently adapted to humans and to the vector A. aegypti. Five genotypes (Angola, Central/East Africa, East Africa, West Africa I, and West Africa II) occur only in Africa. West Africa genotype I is found in Nigeria and the surrounding region.[64] West Africa genotype I appears to be especially infectious, as it is often associated with major outbreaks. The three genotypes found outside of Nigeria and Angola occur in areas where outbreaks are rare. Two outbreaks, in Kenya (1992–1993) and Sudan (2003 and 2005), involved the East African genotype, which had remained undetected in the previous 40 years.[65]
South America

In South America, two genotypes have been identified (South American genotypes I and II).[10] Based on phylogenetic analysis these two genotypes appear to have originated in West Africa[66] and were first introduced into Brazil.[67] The date of introduction of the predecessor African genotype which gave rise to the South American genotypes appears to be 1822 (95% confidence interval 1701 to 1911).[67] The historical record shows an outbreak of yellow fever occurred in Recife, Brazil, between 1685 and 1690. The disease seems to have disappeared, with the next outbreak occurring in 1849. It was likely introduced with the importation of slaves through the slave trade from Africa. Genotype I has been divided into five subclades, A through E.[68]
In late 2016, a large outbreak began in Minas Gerais state of Brazil that was characterized as a sylvan or jungle epizootic.[69] It began as an outbreak in brown howler monkeys,[70] which serve as a sentinel species for yellow fever, that then spread to men working in the jungle. No cases had been transmitted between humans by the A. aegypti mosquito, which can sustain urban outbreaks that can spread rapidly. In April 2017, the sylvan outbreak continued moving toward the Brazilian coast, where most people were unvaccinated.[71] By the end of May the outbreak appeared to be declining after more than 3,000 suspected cases, 758 confirmed and 264 deaths confirmed to be yellow fever.[72] The Health Ministry launched a vaccination campaign and was concerned about spread during the Carnival season in February and March.[73] The CDC issued a Level 2 alert (practice enhanced precautions.)[74]
A Bayesian analysis of genotypes I and II has shown that genotype I accounts for virtually all the current infections in Brazil, Colombia, Venezuela, and Trinidad and Tobago, while genotype II accounted for all cases in Peru.[75] Genotype I originated in the northern Brazilian region around 1908 (95% highest posterior density interval [HPD]: 1870–1936). Genotype II originated in Peru in 1920 (95% HPD: 1867–1958). The estimated rate of mutation for both genotypes was about 5 × 10−4 substitutions/site/year, similar to that of other RNA viruses.[75]
Asia
The main vector, A. aegypti also occurs in tropical and subtropical regions of Asia, the Pacific, and Australia, but yellow fever has never occurred there, until jet travel introduced 11 cases from the 2016 Angola and DR Congo yellow fever outbreak in Africa[76].[77]
But none is considered satisfactory.[78][79] Another proposal is the absence of a slave trade to Asia on the scale of that to the Americas.[80] The trans-Atlantic slave trade probably introduced yellow fever into the Western Hemisphere from Africa.[81]
History


Early history
The evolutionary origins of yellow fever most likely lie in Africa, with transmission of the disease from nonhuman primates to humans.[82][83] The virus is thought to have originated in East or Central Africa and spread from there to West Africa. As it was endemic in Africa, local populations had developed some immunity to it. When an outbreak of yellow fever would occur in an African community where colonists resided, most Europeans died, while the indigenous Africans usually develop nonlethal symptoms resembling influenza.[84] This phenomenon, in which certain populations develop immunity to yellow fever due to prolonged exposure in their childhood, is known as acquired immunity.[85]
The first definitive outbreak of yellow fever in the New World was in 1647 on the island of Barbados.[86] An outbreak was recorded by Spanish colonists in 1648 in the Yucatán Peninsula, where the indigenous Mayan people called the illness xekik ("blood vomit"). In 1685, Brazil suffered its first epidemic in Recife. The first mention of the disease by the name "yellow fever" occurred in 1744.[87] McNeill argues that the environmental and ecological disruption caused by the introduction of sugar plantations created the conditions for mosquito and viral reproduction, and subsequent outbreaks of yellow fever.[88]

In Colonial times and during the Napoleonic Wars, the West Indies were known as a particularly dangerous posting for soldiers due to yellow fever being endemic in the area. The mortality rate in British garrisons in Jamaica was seven times that of garrisons in Canada, mostly because of yellow fever and other tropical diseases.[89] Both English and French forces posted there were seriously affected by the "yellow jack." Wanting to regain control of the lucrative sugar trade in Saint-Domingue (Hispaniola), and with an eye on regaining France's New World empire, Napoleon sent an army under the command of his brother-in-law General Charles Leclerc to Saint-Domingue to seize control after a slave revolt. The historian J. R. McNeill asserts that yellow fever accounted for about 35,000 to 45,000 casualties of these forces during the fighting.[90] Only one third of the French troops survived for withdrawal and return to France. Napoleon gave up on the island and his plans for North America, selling the Louisiana Purchase to the US in 1803. In 1804, Haiti proclaimed its independence as the second republic in the Western Hemisphere. Considerable debate exists over whether the number of deaths caused by disease in the Haitian Revolution was exaggerated.[91]
Although yellow fever is most prevalent in tropical-like climates, the northern United States were not exempted from the fever. The first outbreak in English-speaking North America occurred in New York City in 1668. English colonists in Philadelphia and the French in the Mississippi River Valley recorded major outbreaks in 1669, as well as additional yellow fever epidemics in Philadelphia, Baltimore, and New York City in the 18th and 19th centuries. The disease traveled along steamboat routes from New Orleans, causing some 100,000–150,000 deaths in total.[92] The yellow fever epidemic of 1793 in Philadelphia, which was then the capital of the United States, resulted in the deaths of several thousand people, more than 9% of the population.[93] The national government fled the city, including President George Washington.[94]

The southern city of New Orleans was plagued with major epidemics during the 19th century, most notably in 1833 and 1853. A major epidemic occurred in both New Orleans and Shreveport, Louisiana in 1873. Its residents called the disease "yellow jack." Urban epidemics continued in the United States until 1905, with the last outbreak affecting New Orleans.[95][10][96]
At least 25 major outbreaks took place in the Americas during the 18th and 19th centuries, including particularly serious ones in Cartagena, Chile, in 1741; Cuba in 1762 and 1900; Santo Domingo in 1803; and Memphis, Tennessee, in 1878.[97]
In the early nineteenth century, the prevalence of yellow fever in the Caribbean "led to serious health problems" and alarmed the United States Navy as numerous deaths and sickness curtailed naval operations and destroyed morale.[98] A tragic episode began in April 1822 when the frigate USS Macedonian left Boston and became part of Commodore James Biddle's West India Squadron. Secretary of the Navy Smith Thompson had assigned the squadron to guard United States merchant shipping and suppress piracy. During their time on deployment from 26 May to 3 August 1822, seventy-six of the Macedonian's officers and men died, including Dr. John Cadle, Surgeon USN. Seventy-four of these deaths were attributed to yellow fever. Biddle reported that another fifty-two of his crew were on sick-list. In their report to the Secretary of the Navy, Biddle and Surgeon's Mate Dr. Charles Chase stated the cause as "fever." As a consequence of this loss, Biddle noted that his squadron was forced to return to Norfolk Navy Yard early. Upon arrival, the Macedonian's crew were provided medical care and quarantined at Craney Island, Virginia.[99][100]
In 1853, Cloutierville, Louisiana, had a late-summer outbreak of yellow fever that quickly killed 68 of the 91 inhabitants. A local doctor concluded that some unspecified infectious agent had arrived in a package from New Orleans.[101][102] In 1854, 650 residents of Savannah, Georgia died from yellow fever.[103] In 1858, St. Matthew's German Evangelical Lutheran Church in Charleston, South Carolina, suffered 308 yellow fever deaths, reducing the congregation by half.[104] A ship carrying persons infected with the virus arrived in Hampton Roads in southeastern Virginia in June 1855.[105] The disease spread quickly through the community, eventually killing over 3,000 people, mostly residents of Norfolk and Portsmouth.[106] In 1873, Shreveport, Louisiana, lost 759 citizens in an 80-day period to a yellow fever epidemic, with over 400 additional victims eventually succumbing. The total death toll from August through November was approximately 1,200.[107][108]
In 1878, about 20,000 people died in a widespread epidemic in the Mississippi River Valley.[109] That year, Memphis had an unusually large amount of rain, which led to an increase in the mosquito population. The result was a huge epidemic of yellow fever.[110] The steamship John D. Porter took people fleeing Memphis northward in hopes of escaping the disease, but passengers were not allowed to disembark due to concerns of spreading yellow fever. The ship roamed the Mississippi River for the next two months before unloading her passengers.[111]
Major outbreaks have also occurred in southern Europe. Gibraltar lost many lives to outbreaks in 1804, 1814, and 1828.[112] Barcelona suffered the loss of several thousand citizens during an outbreak in 1821. The Duke de Richelieu deployed 30,000 French troops to the border between France and Spain in the Pyrenees Mountains, to establish a cordon sanitaire in order to prevent the epidemic from spreading from Spain into France.[113]
Proposed causes
Ezekiel Stone Wiggins, known as the Ottawa Prophet, proposed that the cause of a yellow fever epidemic in Jacksonville, Florida, in 1888, was astrological.
The planets were in the same line as the sun and earth and this produced, besides Cyclones, Earthquakes, etc., a denser atmosphere holding more carbon and creating microbes. Mars had an uncommonly dense atmosphere, but its inhabitants were probably protected from the fever by their newly discovered canals, which were perhaps made to absorb carbon and prevent the disease.[114]

In 1848, Josiah C. Nott suggested that yellow fever was spread by insects such as moths or mosquitoes, basing his ideas on the pattern of transmission of the disease.[115] Carlos Finlay, a Cuban doctor and scientist, proposed in 1881 that yellow fever might be transmitted by mosquitoes rather than direct human contact.[116][117] Since the losses from yellow fever in the Spanish–American War in the 1890s were extremely high, Army doctors began research experiments with a team led by Walter Reed, and composed of doctors James Carroll, Aristides Agramonte, and Jesse William Lazear. They successfully proved Finlay's ″mosquito hypothesis″. Yellow fever was the first virus shown to be transmitted by mosquitoes. The physician William Gorgas applied these insights and eradicated yellow fever from Havana. He also campaigned against yellow fever during the construction of the Panama Canal. A previous effort of canal building by the French had failed in part due to mortality from the high incidence of yellow fever and malaria, which killed many workers.[10]
Although Dr. Walter Reed has received much of the credit in United States history books for "beating" yellow fever, he had fully credited Dr. Finlay with the discovery of the yellow fever vector, and how it might be controlled. Reed often cited Finlay's papers in his own articles, and also credited him for the discovery in his personal correspondence.[118] The acceptance of Finlay's work was one of the most important and far-reaching effects of the U.S. Army Yellow Fever Commission of 1900.[119] Applying methods first suggested by Finlay, the United States government and Army eradicated yellow fever in Cuba and later in Panama, allowing completion of the Panama Canal. While Reed built on the research of Finlay, historian François Delaporte notes that yellow fever research was a contentious issue. Scientists, including Finlay and Reed, became successful by building on the work of less prominent scientists, without always giving them the credit they were due.[120] Reed's research was essential in the fight against yellow fever. He is also credited for using the first type of medical consent form during his experiments in Cuba, an attempt to ensure that participants knew they were taking a risk by being part of testing.[121]

Like Cuba and Panama, Brazil also led a highly successful sanitation campaign against mosquitoes and yellow fever. Beginning in 1903, the campaign led by Oswaldo Cruz, then director general of public health, resulted not only in eradicating the disease but also in reshaping the physical landscape of Brazilian cities such as Rio de Janeiro. During rainy seasons, Rio de Janeiro had regularly suffered floods, as water from the bay surrounding the city overflowed into Rio's narrow streets. Coupled with the poor drainage systems found throughout Rio, this created swampy conditions in the city's neighborhoods. Pools of stagnant water stood year-long in city streets and proved to be a fertile ground for disease-carrying mosquitoes. Thus, under Cruz's direction, public health units known as "mosquito inspectors" fiercely worked to combat yellow fever throughout Rio by spraying, exterminating rats, improving drainage, and destroying unsanitary housing. Ultimately, the city's sanitation and renovation campaigns reshaped Rio de Janeiro's neighborhoods. Its poor residents were pushed from city centers to Rio's suburbs, or to towns found in the outskirts of the city. In later years, Rio's most impoverished inhabitants would come to reside in favelas.[122]
During 1920–23, the Rockefeller Foundation’s International Health Board undertook an expensive and successful yellow fever eradication campaign in Mexico.[123] The IHB gained the respect of Mexico's federal government because of the success. The eradication of yellow fever strengthened the relationship between the US and Mexico, which had not been very good in the years prior. The eradication of yellow fever was also a major step toward better global health.[124]
In 1927, scientists isolated yellow fever virus in West Africa.[125] Following this, two vaccines were developed in the 1930s. Vaccine 17D is still in use although newer vaccines, based on vero cells, are in development.[4][126][127]
Current status
Using vector control and strict vaccination programs, the urban cycle of yellow fever was nearly eradicated from South America. Since 1943, only a single urban outbreak in Santa Cruz de la Sierra, Bolivia, has occurred. Since the 1980s, however, the number of yellow fever cases has been increasing again, and A. aegypti has returned to the urban centers of South America. This is partly due to limitations on available insecticides, as well as habitat dislocations caused by climate change. It is also because the vector control program was abandoned. Although no new urban cycle has yet been established, scientists believe this could happen again at any point. An outbreak in Paraguay in 2008 was thought to be urban in nature, but this ultimately proved not to be the case.[4]
In Africa, virus eradication programs have mostly relied upon vaccination. These programs have largely been unsuccessful because they were unable to break the sylvatic cycle involving wild primates. With few countries establishing regular vaccination programs, measures to fight yellow fever have been neglected, making the future spread of the virus more likely.[4]
Research
In the hamster model of yellow fever, early administration of the antiviral ribavirin is an effective treatment of many pathological features of the disease.[128] Ribavirin treatment during the first five days after virus infection improved survival rates, reduced tissue damage in the liver and spleen, prevented hepatocellular steatosis, and normalised levels of alanine aminotransferase, a liver damage marker. The mechanism of action of ribavirin in reducing liver pathology in yellow fever virus infection may be similar to its activity in treatment of hepatitis C, a related virus.[128] Because ribavirin had failed to improve survival in a virulent rhesus model of yellow fever infection, it had been previously discounted as a possible therapy.[129] Infection was reduced in mosquitoes with the wMel strain of Wolbachia.[130]Yellow fever has been researched by several countries as a potential biological weapon.[131]
References
- ↑ 1.0 1.1 1.2 1.3 Oldstone, Michael (2009). Viruses, Plagues, and History: Past, Present and Future. Oxford University Press. pp. 102–4. ISBN 978-0-19-975849-4. Archived from the original on 2017-02-23.
- ↑ Bazin, Hervé (2011). Vaccination: a history from Lady Montagu to genetic engineering. Montrouge: J. Libbey Eurotext. p. 407. ISBN 978-2-7420-0775-2. Archived from the original on 2017-02-23.
- ↑ 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 3.11 3.12 3.13 3.14 3.15 3.16 3.17 3.18 3.19 3.20 3.21 3.22 3.23 3.24 3.25 3.26 "Yellow fever Fact sheet N°100". World Health Organization. May 2013. Archived from the original on 19 February 2014. Retrieved 23 February 2014.
- ↑ 4.00 4.01 4.02 4.03 4.04 4.05 4.06 4.07 4.08 4.09 4.10 4.11 4.12 Tolle MA (April 2009). "Mosquito-borne diseases". Curr Probl Pediatr Adolesc Health Care. 39 (4): 97–140. doi:10.1016/j.cppeds.2009.01.001. PMID 19327647.
- ↑ Wang, Haidong; et al. (8 October 2016). "Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980 – 2015: a systematic analysis for the Global Burden of Disease Study 2015". The Lancet. 388 (10053): 1459–1544. doi:10.1016/s0140-6736(16)31012-1. PMC 5388903. PMID 27733281.
- ↑ Scully, Crispian (2014). Scully's Medical Problems in Dentistry. Elsevier Health Sciences. p. 572. ISBN 978-0-7020-5963-6. Archived from the original on 2020-11-22. Retrieved 2020-07-30.
- ↑ 7.0 7.1 7.2 Lindenbach, B. D.; et al. (2007). "Flaviviridae: The Viruses and Their Replication". In Knipe, D. M.; P. M. Howley (eds.). Fields Virology (5th ed.). Philadelphia, PA: Lippincott Williams & Wilkins. p. 1101. ISBN 978-0-7817-6060-7.
- ↑ 8.0 8.1 "Frequently Asked Questions About Yellow Fever". CDC. August 21, 2015. Archived from the original on 23 March 2016. Retrieved 18 March 2016.
- ↑ "CDC Yellow Fever". Archived from the original on 2012-12-21. Retrieved 2012-12-12.
- ↑ 10.0 10.1 10.2 10.3 10.4 10.5 Barrett AD, Higgs S (2007). "Yellow fever: a disease that has yet to be conquered". Annu. Rev. Entomol. 52: 209–29. doi:10.1146/annurev.ento.52.110405.091454. PMID 16913829. S2CID 9896455. Archived from the original on 2021-04-29. Retrieved 2020-07-30.
- ↑ Sfakianos, Jeffrey; Hecht, Alan (2009). Babcock, Hilary (ed.). West Nile Virus (Curriculum-based juvenile nonfiction). Deadly Diseases & Epidemics. Foreword by David Heymann (2nd ed.). New York: Chelsea House. p. 17. ISBN 978-1-60413-254-0. Archived from the original on 2021-01-26. Retrieved 2020-07-30.
The yellow fever virus was isolated in 1927
- ↑ "CDC: Yellow fever—Symptoms and treatment". Archived from the original on 2012-03-21. Retrieved 2010-11-10.
- ↑ "Yellow fever". WHO. Archived from the original on 2009-08-17. Retrieved 2009-08-13.
- ↑ Control of Communicable Diseases Manual (20th ed.). Amer Public Health Assn. 2015. ISBN 978-0-87553-018-5.
- ↑ Chastel C (August 2003). "[Centenary of the discovery of yellow fever virus and its transmission by a mosquito (Cuba 1900–1901)]". Bull Soc Pathol Exot (in French). 96 (3): 250–6. PMID 14582304.
{{cite journal}}: CS1 maint: unrecognized language (link) - ↑ Dr. Irwin Sherman "Twelve Diseases that Changed Our World". P. 144. ASM Press. 2007. ISBN 978-1-55581-466-3. OCLC 141178241.
- ↑ Franklin, Jon; Sutherland, John. "Guinea Pig Doctors: The Drama of Medical Research Through Self-Experimentation" by Jon Franklin (Author), John Sutherland (Author) Publisher: William Morrow & Co (March 1984) ISBN 0-688-02666-4
- ↑ 18.0 18.1 18.2 18.3 Monath TP (April 2008). "Treatment of yellow fever". Antiviral Res. 78 (1): 116–24. doi:10.1016/j.antiviral.2007.10.009. PMID 18061688. S2CID 44119619. Archived from the original on 2021-08-27. Retrieved 2020-07-30.
- ↑ Tomori O (2004). "Yellow fever: the recurring plague". Crit Rev Clin Lab Sci. 41 (4): 391–427. doi:10.1080/10408360490497474. PMID 15487593. S2CID 13559157.
- ↑ Modrow, S.; et al. (2002). Molekulare Virologie – Eine Einführung für Biologen und Mediziner (2nd ed.). Spektrum Akademischer Verlag. p. 182. ISBN 978-3-8274-1086-3.
- ↑ Rogers DJ, Wilson AJ, Hay SI, Graham AJ (2006). "The global distribution of yellow fever and dengue". Global Mapping of Infectious Diseases: Methods, Examples and Emerging Applications. Adv. Parasitol. Advances in Parasitology. Vol. 62. pp. 181–220. doi:10.1016/S0065-308X(05)62006-4. ISBN 978-0-12-031762-2. PMC 3164798. PMID 16647971.
- ↑ Staples JE, Monath TP (Aug 27, 2008). "Yellow fever: 100 years of discovery". JAMA: The Journal of the American Medical Association. 300 (8): 960–2. doi:10.1001/jama.300.8.960. PMID 18728272.
- ↑ 23.0 23.1 23.2 Sampath A, Padmanabhan R (January 2009). "Molecular targets for flavivirus drug discovery". Antiviral Research. 81 (1): 6–15. doi:10.1016/j.antiviral.2008.08.004. PMC 2647018. PMID 18796313.
- ↑ Silva, Patricia A. G. C. (2010). "An RNA Pseudoknot Is Required for Production of Yellow Fever Virus Subgenomic RNA by the Host Nuclease XRN1". Journal of Virology. 84 (21): 11395–11406. doi:10.1128/jvi.01047-10. PMC 2953177. PMID 20739539.
- ↑ 25.0 25.1 25.2 25.3 25.4 Mangat, Rupinder; Louie, Ted (2022). "Viral Hemorrhagic Fevers". StatPearls. StatPearls Publishing. Archived from the original on 11 January 2022. Retrieved 27 February 2022.
- ↑ Dhiman, Gaurav.; Abraham, R.; Griffin, D. (2019). "Human Schwann cells are susceptible to infection with Zika and yellow fever viruses, but not dengue virus". Scientific Reports. 9 (1): 9951. Bibcode:2019NatSR...9.9951D. doi:10.1038/s41598-019-46389-0. PMC 6616448. PMID 31289325.
- ↑ Fontenille D, Diallo M, Mondo M, Ndiaye M, Thonnon J (1997). "First evidence of natural vertical transmission of yellow fever virus in Aedes aegypti, its epidemic vector". Transactions of the Royal Society of Tropical Medicine and Hygiene. 91 (5): 533–5. doi:10.1016/S0035-9203(97)90013-4. PMID 9463659.
{{cite journal}}: CS1 maint: uses authors parameter (link) - ↑ "Infectious Diseases Related to Travel". Yellow Book. Centers for Disease Control and Prevention. Archived from the original on 20 March 2016. Retrieved 20 March 2016.
- ↑ Paixão, Enny S.; Teixeira, Maria Gloria; Rodrigues, Laura C (4 January 2018). "Zika, chikungunya and dengue: the causes and threats of new and re-emerging arboviral diseases". BMJ Global Health. 3 (Suppl 1): e000530. doi:10.1136/bmjgh-2017-000530. ISSN 2059-7908. Archived from the original on 21 January 2022. Retrieved 1 April 2022.
- ↑ "Transmission of Yellow Fever Virus". www.cdc.gov. 16 January 2019. Archived from the original on 30 March 2022. Retrieved 2 April 2022.
- ↑ "Yellow fever fact sheet". WHO—Yellow fever. Archived from the original on 2006-04-18. Retrieved 2006-04-18.
- ↑ "Ebola outbreak Alert and response operations Diseases Biorisk reduction Yellow fever : a current threat". WHO. Archived from the original on 8 August 2016. Retrieved 4 August 2016.
- ↑ de Vries, Liset; Harding, Alfred T. (February 2023). "Mechanisms of Neuroinvasion and Neuropathogenesis by Pathologic Flaviviruses". Viruses. 15 (2): 261. doi:10.3390/v15020261. ISSN 1999-4915.
- ↑ Ryan, K. J.; C. G. Ray., eds. (2004). Sherris Medical Microbiology (4th ed.). McGraw Hill. ISBN 978-0-8385-8529-0.
- ↑ Quaresma JA, Barros VL, Pagliari C, Fernandes ER, Guedes F, Takakura CF, Andrade HF, Vasconcelos PF, Duarte MI (2006). "Revisiting the liver in human yellow fever: virus-induced apoptosis in hepatocytes associated with TGF-beta, TNF-alpha and NK cells activity". Virology. 345 (1): 22–30. doi:10.1016/j.virol.2005.09.058. PMID 16278000.
- ↑ 36.0 36.1 "Diagnostic Testing | Yellow Fever | CDC". www.cdc.gov. 15 January 2019. Archived from the original on 25 March 2022. Retrieved 31 March 2022.
- ↑ 37.0 37.1 "Laboratory diagnosis of yellow fever virus infection". apo.who.int. Archived from the original on 3 April 2022. Retrieved 2 April 2022.
- ↑ "Laboratory Diagnosis of Yellow Fever Virus infection" (PDF). WHO.int. WHO. Archived (PDF) from the original on 22 October 2020. Retrieved 6 April 2022.
- ↑ "Yellow Fever Differential Diagnoses". emedicine.medscape.com. Archived from the original on 2 December 2020. Retrieved 3 April 2022.
- ↑ 40.0 40.1 40.2 40.3 "Prevention | Yellow Fever | CDC". www.cdc.gov. Archived from the original on 2016-10-26. Retrieved 2016-10-26.
- ↑ 41.0 41.1 "Prevent Tick and Mosquito Bites | Division of Vector-Borne Diseases | NCEZID | CDC". www.cdc.gov. 9 February 2022. Archived from the original on 5 March 2022. Retrieved 16 April 2022.
- ↑ 42.0 42.1 42.2 42.3 Barrett AD, Teuwen DE (June 2009). "Yellow fever vaccine – how does it work and why do rare cases of serious adverse events take place?". Current Opinion in Immunology. 21 (3): 308–13. doi:10.1016/j.coi.2009.05.018. PMID 19520559.
- ↑ WHO | Yellow fever vaccination booster not needed Archived 2013-06-09 at the Wayback Machine. Who.int (2013-05-17). Retrieved on 2014-05-12.
- ↑ Yellow Fever Vaccine Information Statement. Archived 2013-09-21 at the Wayback Machine Centers for Disease Control and Prevention. March 30, 2011.
- ↑ "Yellow fever". World Health Organization. Archived from the original on 18 April 2017. Retrieved 2 April 2017.
- ↑ "Twelve million West Africans get yellow fever vaccines". BBC News. 23 November 2009. Archived from the original on 8 September 2017. Retrieved 23 November 2009.
- ↑ 47.0 47.1 47.2 47.3 47.4 "Fractional Dose Yellow Fever Vaccine as a Dose-sparing Option for Outbreak Response. WHO Secretariat Information Paper. Department of Immunization, Vaccines and Biologicals. WHO reference number: WHO/YF/SAGE/16.1". World Health Organization. 20 July 2016. Archived from the original on 10 January 2020. Retrieved 2 September 2018.
- ↑ "WHO supports the immunization of 874 000 people against yellow fever in Nigeria. News Release". World Health Organization. 16 October 2017. Archived from the original on 18 April 2018. Retrieved 2 September 2018.
- ↑ World Health Organization (WHO). WHO position on the use of fractional doses – June 2017, addendum to vaccines and vaccination against yellow fever WHO: Position paper – June 2013. Vaccine 2017;35(43):5751-52. doi: 10.1016/j.vaccine.2017.06.087 [published Online First: 2017/07/12]
- ↑ "WHO dispatched 3.5 million doses of yellow fever vaccine for outbreak response in Brazil". World Health Organization. Archived from the original on 1 April 2017. Retrieved 2 April 2017.
- ↑ "Yellow fever – Brazil". Nature. 150 (3811): 573. 1942. Bibcode:1942Natur.150T.573.. doi:10.1038/150573d0. Archived from the original on 6 March 2017. Retrieved 9 March 2017.
- ↑ Darlington, Shasta (20 March 2018). "Fearing New Outbreaks, Brazil Will Vaccinate Country Against Yellow Fever". The New York Times. Archived from the original on 21 March 2018. Retrieved 21 March 2018.
- ↑ "Country list: Yellow fever vaccination requirements and recommendations; malaria situation; and other vaccination requirements" (PDF). WHO. 2013. p. 32. Archived (PDF) from the original on 2015-04-05. Retrieved 2015-04-12.
- ↑ Hansen, Immo A.; Boudko, Dmitri Y.; Shiao, Shin-Hong; Voronov, Dmitri A.; Meleshkevitch, Ella A.; Drake, Lisa L.; Aguirre, Sarah E.; Fox, Jeffrey M.; Attardo, Geoffrey M.; Raikhel, Alexander S. (March 2011). "AaCAT1 of the Yellow Fever Mosquito, Aedes aegypti". Journal of Biological Chemistry. 286 (12): 10803–10813. doi:10.1074/jbc.M110.179739. Archived from the original on 1 April 2022. Retrieved 31 March 2022.
- ↑ Wilson, Anne L.; Courtenay, Orin; Kelly-Hope, Louise A.; Scott, Thomas W.; Takken, Willem; Torr, Steve J.; Lindsay, Steve W. (16 January 2020). "The importance of vector control for the control and elimination of vector-borne diseases". PLoS Neglected Tropical Diseases. 14 (1): e0007831. doi:10.1371/journal.pntd.0007831. ISSN 1935-2727. Archived from the original on 10 February 2022. Retrieved 4 April 2022.
- ↑ "Frequently asked questions:Yellow fever". Florida Dept. of Health/view.officeapps.live.com. FDOH. Retrieved 11 April 2022.
- ↑ "Yellow Fever Treatment & Management: Approach Considerations, Emergency Department Care, Deterrence and Prevention". MEDSCAPE. 16 October 2021. Archived from the original on 25 June 2021. Retrieved 1 April 2022.
- ↑ Rollins, Daniel; Ramsey, Renee; Parsh, Bridget (September 2017). "Yellow fever". Nursing2022. 47 (9): 69–70. doi:10.1097/01.NURSE.0000522022.53547.ed. ISSN 0360-4039. Retrieved 1 April 2022.
- ↑ Barnett, Elizabeth D. (15 March 2007). "Yellow fever: epidemiology and prevention". Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America. 44 (6): 850–856. doi:10.1086/511869. ISSN 1537-6591. Archived from the original on 2 March 2022. Retrieved 3 April 2022.
- ↑ Gianchecchi, Elena; Cianchi, Virginia; Torelli, Alessandro; Montomoli, Emanuele (27 February 2022). "Yellow Fever: Origin, Epidemiology, Preventive Strategies and Future Prospects". Vaccines. 10 (3): 372. doi:10.3390/vaccines10030372. ISSN 2076-393X. Archived from the original on 5 April 2022. Retrieved 5 April 2022.
- ↑ "Global Health - Newsroom - Yellow Fever". www.cdc.gov. 19 February 2019. Archived from the original on 11 February 2022. Retrieved 12 April 2022.
- ↑ "Yellow Fever – China". World Health Organization. Archived from the original on 19 March 2017. Retrieved 9 February 2017.
- ↑ Woodall, J.P.; Yuill, T.M. (July 2016). "Why is the yellow fever outbreak in Angola a 'threat to the entire world'?". International Journal of Infectious Diseases. 48: 96–97. doi:10.1016/j.ijid.2016.05.001. PMID 27163382.
- ↑ Mutebi JP, Barrett AD (2002). "The epidemiology of yellow fever in Africa". Microbes Infect. 4 (14): 1459–1468. doi:10.1016/S1286-4579(02)00028-X. PMID 12475636.
- ↑ Ellis BR, Barrett AD (2008). "The enigma of yellow fever in East Africa". Rev Med Virol. 18 (5): 331–346. doi:10.1002/rmv.584. PMID 18615782. S2CID 23266086.
- ↑ Mutebi JP, Rijnbrand RC, Wang H, Ryman KD, Wang E, Fulop LD, Titball R, Barrett AD (2004). "Genetic relationships and evolution of genotypes of yellow fever virus and other members of the yellow fever virus group within the genus Flavivirus based on the 3' noncoding region". J Virol. 78 (18): 9652–9665. doi:10.1128/JVI.78.18.9652-9665.2004. PMC 515011. PMID 15331698.
- ↑ 67.0 67.1 Auguste AJ, Lemey P, Pybus OG, Suchard MA, Salas RA, Adesiyun AA, Barrett AD, Tesh RB, Weaver SC, Carrington CV (2010). "Yellow fever virus maintenance in Trinidad and its dispersal throughout the Americas". J Virol. 84 (19): 9967–9977. doi:10.1128/JVI.00588-10. PMC 2937779. PMID 20631128.
- ↑ de Souza RP, Foster PG, Sallum MA, Coimbra TL, Maeda AY, Silveira VR, Moreno ES, da Silva FG, Rocco IM, Ferreira IB, Suzuki A, Oshiro FM, Petrella SM, Pereira LE, Katz G, Tengan CH, Siciliano MM, Dos Santos CL (2010). "Detection of a new yellow fever virus lineage within the South American genotype I in Brazil". J Med Virol. 82 (1): 175–185. doi:10.1002/jmv.21606. PMID 19950229. S2CID 96746.
- ↑ "YELLOW FEVER - AMERICAS (01): PAHO/WHO 2016". www.promedmail.org. International Society for Infectious Diseases. Archived from the original on 16 February 2017. Retrieved 16 February 2017.
- ↑ "Yellow fever killing thousands of monkeys in Brazil". www.sciencedaily.com. Archived from the original on 24 March 2017. Retrieved 24 March 2017.
- ↑ "Yellow fever – Brazil". Nature. 150 (3811): 573. 1942. Bibcode:1942Natur.150T.573.. doi:10.1038/150573d0. Archived from the original on 9 April 2017. Retrieved 9 April 2017.
- ↑ "ProMED-mail post Yellow fever - Americas (47): Brazil, PAHO/WHO". www.promedmail.org. International Society for Infectious Diseases. Archived from the original on 8 September 2017. Retrieved 1 June 2017.
- ↑ Times, Global. "Yellow fever cases continue to rise in Brazil, 253 confirmed – Global Times". www.globaltimes.cn. Archived from the original on 19 February 2017. Retrieved 19 February 2017.
- ↑ "Yellow Fever in Brazil – Alert – Level 2, Practice Enhanced Precautions – Travel Health Notices | Travelers' Health | CDC". wwwnc.cdc.gov. Archived from the original on 25 May 2017. Retrieved 1 June 2017.
- ↑ 75.0 75.1 Mir, Daiana; Delatorre, Edson; Bonaldo, Myrna; Lourenço-de-Oliveira, Ricardo; Vicente, Ana Carolina; Bello, Gonzalo (7 August 2017). "Phylodynamics of Yellow Fever Virus in the Americas: new insights into the origin of the 2017 Brazilian outbreak". Scientific Reports. 7 (1): 7385. doi:10.1038/s41598-017-07873-7. ISSN 2045-2322. Archived from the original on 4 March 2022. Retrieved 4 April 2022.
- ↑ Li, Chao; Li, Dan; Smart, Shirley JoAnn; Zhou, Lei; Yang, Peng; Ou, Jianming; He, Yi; Ren, Ruiqi; Ma, Tao; Xiang, Nijuan; Sui, Haitian; Wang, Yali; Zhao, Jian; Wang, Chaonan; Wang, Yeping; Ni, Daxin; Fung, Isaac Chun-Hai; Li, Dexin; Huang, Yangmu; Li, Qun (30 June 2020). "Evaluating the importation of yellow fever cases into China in 2016 and strategies used to prevent and control the spread of the disease". Western Pacific Surveillance and Response Journal : WPSAR. 11 (2): 5–10. doi:10.5365/wpsar.2018.9.1.007. ISSN 2094-7321. Archived from the original on 13 April 2022. Retrieved 13 April 2022.
- ↑ "Experts Alarmed by Yellow Fever Cases in Asia". Medscape. Archived from the original on 8 October 2017. Retrieved 14 April 2022.
- ↑ Vainio J.; F. Cutts, eds. (1998). Yellow Fever. WHO Division of Emerging and other Communicable Diseases Surveillance and Control.
- ↑ Monath, T. P. (1989). "The absence of yellow fever in Asia: hypotheses. A cause for concern?". Virus Inf Exch Newslett: 106–7.
- ↑ Cathey JT, Marr JS (2014). "Yellow fever, Asia and the East African slave trade". Trans R Soc Trop Med Hyg. 108 (5): 252–7. doi:10.1093/trstmh/tru043. PMID 24743951.
- ↑ Bryant JE, Holmes EC, Barrett AD (2007). "Out of Africa: a molecular perspective on the introduction of yellow fever virus into the Americas". PLOS Pathog. 3 (5): e75. doi:10.1371/journal.ppat.0030075. PMC 1868956. PMID 17511518.
- ↑ Gould EA, de Lamballerie X, Zanotto PM, Holmes EC (2003). Origins, evolution, coadaptations within the genus Flavivirus. Advances in Virus Research. Vol. 59. pp. 277–314. doi:10.1016/S0065-3527(03)59008-X. ISBN 978-0-12-039859-1. PMID 14696332.
- ↑ Bryant, JE; Holmes, EC; Barrett, AD (18 May 2007). "Out of Africa: a molecular perspective on the introduction of yellow fever virus into the Americas". PLOS Pathogens. 3 (5): e75. doi:10.1371/journal.ppat.0030075. PMC 1868956. PMID 17511518.
- ↑ Oldstone, M. (1998). Viruses, Plagues, and History, New York: Oxford University Press.
- ↑ McNeill, J. R. (2010). Mosquito Empires: Ecology and war in the greater Caribbean, 1620–1914. NY: Cambridge University Press. pp. 44–45.
- ↑ McNeill, J. R. (1 April 2004). "Yellow Jack and Geopolitics: Environment, Epidemics, and the Struggles for Empire in the American Tropics, 1650–1825". OAH Magazine of History. 18 (3): 9–13. doi:10.1093/maghis/18.3.9. Archived from the original on 20 December 2016.
- ↑ The earliest mention of "yellow fever" appears in a manuscript of 1744 by Dr. John Mitchell of Virginia; copies of the manuscript were sent to Mr. Cadwallader Colden, a physician in New York, and to Dr. Benjamin Rush of Philadelphia; the manuscript was eventually printed (in large part) in 1805 and reprinted in 1814. See:
- (John Mitchell) (1805) (Mitchell's account of the Yellow Fever in Virginia in 1741–2) Archived 2017-02-23 at the Wayback Machine, The Philadelphia Medical Museum, 1 (1) : 1–20.
- (John Mitchell) (1814) "Account of the Yellow fever which prevailed in Virginia in the years 1737, 1741, and 1742, in a letter to the late Cadwallader Colden, Esq. of New York, from the late John Mitchell, M.D.F.R.S. of Virginia," Archived 2017-02-23 at the Wayback Machine American Medical and Philosophical Register, 4 : 181–215. The term "yellow fever" appears on p. 186. On p. 188, Mitchell mentions "… the distemper was what is generally called yellow fever in America." However, on pages 191–192, he states "… I shall consider the cause of the yellowness which is so remarkable in this distemper, as to have given it the name of the Yellow Fever."
- ↑ McNeill, John (2010). Mosquito Empires: Ecology and War in the Greater Caribbean, 1620–1914. New York, NY: Cambridge University Press. ISBN 978-0-511-67268-2.
- ↑ McNeill, JR (2002). "Yellow fever and geopolitics: environment, epidemics, and the struggles for empire in the American tropics, 1650–1900". History Now (Christchurch, N.Z.). 8 (2): 10–6. PMID 20690235.
- ↑ McNeill, J.R. (2010). Moaquito Empires: Ecology and War in the Greater Caribbean, 1620–1914. Cambridge University Press. p. 259.
- ↑ Philippe R. Girard (2011). The Slaves Who Defeated Napoleon: Toussaint Louverture and the Haitian War of Independence, 1801–1804. University of Alabama Press. pp. 179–80. ISBN 978-0-8173-1732-4. Archived from the original on 2016-09-11.
- ↑ Patterson, KD (1992). "Yellow fever epidemics and mortality in the United States, 1693–1905". Social Science & Medicine. 34 (8): 855–865. doi:10.1016/0277-9536(92)90255-O. PMID 1604377.
- ↑ Miller, Jacquelyn C (2005). "The Wages of Blackness: African American Workers and the Meanings of Race during Philadelphia's 1793 Yellow Fever Epidemic". The Pennsylvania Magazine of History and Biography. 129 (2): 163–194.
- ↑ "Yellow Fever Attacks Philadelphia, 1793". EyeWitness to History. Archived from the original on 2007-06-07. Retrieved 2009-08-14.
- ↑ John Pierce; Jim Writer (2005). Yellow Jack: How Yellow Fever ravaged America and Walter Reed Discovered Its Deadly Secrets. Hoboken: John Wiley & Sons. p. 3.
- ↑ "The Tennessee Encyclopedia of History and Culture:Yellow Fever Epidemics". Tennessee Historical Society. Archived from the original on December 12, 2013. Retrieved June 20, 2013.
- ↑ John S. Marr, and John T. Cathey. "The 1802 Saint-Domingue yellow fever epidemic and the Louisiana Purchase." Journal of Public Health Management and Practice 19#.1 (2013): 77–82. online Archived 2016-02-04 at the Wayback Machine
- ↑ Langley, Harold D. A History of Medicine in the Early U.S. Navy (Johns Hopkins Press: Baltimore 1995), 274-275
- ↑ NARA M125 volume 79 letter no. 15 "Captains Letters" James Biddle to Smith Thompson 3 August 1822
- ↑ The Macedonian a list of the deaths, Connecticut Herald 20 August 1822, p.2
- ↑ The Transactions of the American Medical Association, Volume IX, TK and PG Collins, 1856, page 704, "Yellow Fever at the Village of Cloutierville, La, in the Years 1853 and 1854" by Samuel O. Scruggs, M.D.
- ↑ New Orleans Genesis June 1970, page 261-262, "Cloutierville Yellow Fever Deaths, 1853"
- ↑ Lockley, Tim (2012). "'Like a clap of thunder in a clear sky': differential mortality during Savannah's yellow fever epidemic of 1854" (PDF). Social History. 37 (2): 166–186. doi:10.1080/03071022.2012.675657. S2CID 2571401. Archived (PDF) from the original on 23 September 2017. Retrieved 22 February 2018.
- ↑ St. Matthew's Evangelical Lutheran Church: 125 Years of Christian Service, 1967.
- ↑ Mauer HB. "Mosquito control ends fatal plague of yellow fever". etext.lib.virginia.edu. Archived from the original on 2012-12-12. Retrieved 2007-06-11. (undated newspaper clipping).
- ↑ "Yellow Fever". www.usgwarchives.net. Archived from the original on 29 September 2019. Retrieved 30 September 2019.
- ↑ "Louisiana Office of Public Health Statistics, page 6" (PDF). Archived (PDF) from the original on 2019-02-04. Retrieved 2020-07-30.
- ↑ "Oakland Cemetery". Archived from the original on 2018-09-28. Retrieved 2020-07-30.
- ↑ Crosby, Molly Caldwell (2006). The American Plague. New York: Berkley Publishing Group. p. 75.
- ↑ "Yellow Fever — the plague of Memphis". HistoricMemphis.com. Archived from the original on August 21, 2014. Retrieved August 20, 2014.
- ↑ Barnes, E. (2005). Diseases and Human Evolution. Albuquerque: University of New Mexico. ISBN 978-0-8263-3065-9.
- ↑ Sawchuk, L. A.; Burke, S. D. A. (1998). "Gibraltar's 1804 Yellow Fever Scourge: The Search for Scapegoats". Journal of the History of Medicine and Allied Sciences. 53 (1): 3–42. doi:10.1093/jhmas/53.1.3. PMID 9510598. Archived from the original on 2011-08-22. Retrieved 2013-04-05.
- ↑ James Taylor, The age we live in: a history of the nineteenth century, Oxford University, 1882; p. 222.
- ↑ John W. Cowart, "Yellow Jack in Jacksonville, Yellow Fever visited Duval County, Florida, in 1888" Archived 2013-01-05 at the Wayback Machine, Historical Text Archive
- ↑ Josiah C. Nott (1848) "Yellow Fever contrasted with Bilious Fever – Reasons for believing it a disease sui generis – Its mode of Propagation – Remote Cause – Probable insect or animalcular origin" Archived 2018-11-19 at the Wayback Machine, The New Orleans Medical and Surgical Journal, "4" : 563–601.
- ↑ Carlos Juan Finlay (presented: August 14, 1881; published: 1882) "El mosquito hipoteticamente considerado como agente de transmission de la fiebre amarilla" Archived 2017-02-23 at the Wayback Machine (The mosquito hypothetically considered as an agent in the transmission of yellow fever) Anales de la Real Academia de Ciencias Médicas, Físicas y Naturales de la Habana, 18 : 147–169. Available on-line in English at:
- Charles Finlay, with Rudolph Matas, translator (1881) "The mosquito hypothetically considered as an agent in the transmission of yellow fever poison," Archived 2017-02-23 at the Wayback Machine New Orleans Medical and Surgical Journal, 9 : 601–616.
- Delta Omega.org Archived 2012-05-09 at the Wayback Machine
- ↑ Chaves-Carballo E (2005). "Carlos Finlay and yellow fever: triumph over adversity". Mil Med. 170 (10): 881–5. doi:10.7205/milmed.170.10.881. PMID 16435764.
- ↑ Pierce, J.R.; Writer, J. (2005). Yellow Jack: How Yellow Fever Ravaged America and Walter Reed Discovered Its Deadly Secrets. Wiley. ISBN 978-0-471-47261-2.
- ↑ "U.S. Army Yellow Fever Commission". UVA Health Sciences: Historical Collections. Archived from the original on 2017-04-26. Retrieved 2017-08-01.
- ↑ Delaporte, Francois (1991). The History of Yellow Fever: An Essay on the Birth of Tropical Medicine. Cambridge: MIT Press. pp. 89–90. ISBN 9780262041126.
- ↑ Crosby, Molly Caldwell (2006). The American Plague. New York: Berkley Publishing Group. p. 177.
- ↑ Teresa A. Meade, A History of Modern Latin America: 1800 To The Present, 1st ed. (Chichester: Wiley-Blackwell, 2010), pp. 148–149.
- ↑ Fosdick, Raymond B. (1952). The Story of the Rockefeller Foundation. New York: Harper & Brothers. pp. 58–79.
- ↑ Birn AE, Solórzano A (1999). "Public health policy paradoxes: science and politics in the Rockefeller Foundation's hookworm campaign in Mexico in the 1920s". Soc Sci Med. 49 (9): 1197–213. doi:10.1016/s0277-9536(99)00160-4. PMID 10501641.
- ↑ Bigon, L. (2014). "Transnational Networks of Administrating Disease and Urban Planning in West Africa: The Inter-Colonial Conference on Yellow Fever, Dakar, 1928". GeoJournal. 79 (1): 103–111. doi:10.1007/s10708-013-9476-z. S2CID 153603689. Archived from the original on 2021-08-27. Retrieved 2020-07-30.
- ↑ National Institutes of Health (July 27, 2016). "NIH launches early-stage yellow fever vaccine trial" (Press release). United States Department of Health and Human Services. Archived from the original on August 26, 2016. Retrieved July 14, 2019.
- ↑ National Institute of Allergy and Infectious Diseases (NIAID) (June 1, 2018), A Phase I Trial to Evaluate the Safety, Reactogenicity, and Immunogenicity of MVA-BN Yellow Fever Vaccine With and Without Montanide ISA-720 Adjuvant in 18–45 Year Old Healthy Volunteers (NCT number: NCT02743455), United States National Library of Medicine, archived from the original on May 12, 2019, retrieved July 14, 2019.
- ↑ 128.0 128.1 Sbrana E, Xiao SY, Guzman H, Ye M, Travassos da Rosa AP, Tesh RB (2004). "Efficacy of post-exposure treatment of yellow fever with ribavirin in a hamster model of the disease". Am J Trop Med Hyg. 71 (3): 306–12. doi:10.4269/ajtmh.2004.71.306. PMID 15381811.
- ↑ Huggins JW (1989). "Prospects for treatment of viral hemorrhagic fevers with ribavirin, a broad-spectrum antiviral drug". Rev Infect Dis. 11 (Suppl 4): S750–61. doi:10.1093/clinids/11.Supplement_4.S750. PMID 2546248.
- ↑ Van Den Hurk, Andrew F.; Hall-Mendelin, Sonja; Pyke, Alyssa T.; Frentiu, Francesca D.; McElroy, Kate; Day, Andrew; Higgs, Stephen; O'Neill, Scott L. (2012). "Impact of Wolbachia on Infection with Chikungunya and Yellow Fever Viruses in the Mosquito Vector Aedes aegypti". PLOS Neglected Tropical Diseases. 6 (11): e1892. doi:10.1371/journal.pntd.0001892. PMC 3486898. PMID 23133693.
- ↑ Endicott, S. L.; Hageman, E. (1998). The United States and Biological Warfare: Secrets from the Early Cold War and Korea. Indiana University Press. ISBN 978-0-253-33472-5.
Further reading
| Library resources about Yellow fever |
- Crosby, M. (2006). The American Plague: The Untold Story of Yellow Fever, the Epidemic that Shaped Our History. New York: The Berkley Publishing Group. ISBN 978-0-425-21202-8.
- Espinosa, M. (2009). Epidemic Invasions: Yellow Fever and the Limits of Cuban Independence, 1878–1930. Chicago: University of Chicago Press. ISBN 978-0-226-21811-3. Archived from the original on 2021-01-26. Retrieved 2020-07-30.
- Gessner, I. (2016). Yellow Fever Years: An Epidemiology of Nineteenth-Century American Literature and Culture. Frankfurt/Main: Peter Lang. ISBN 978-3-631-67412-3.
- Murphy, J. (2003). An American Plague: The True and Terrifying Story of the Yellow Fever Epidemic of 1793. New York: Clarion Books. ISBN 978-0-395-77608-7.
- Nuwer, D. S. (2009). Plague Among the Magnolias: The 1878 Yellow Fever Epidemic in Mississippi. University of Alabama Press. ISBN 978-0-8173-1653-2.
- "Yellow Fever - Office of NIH History and Stetten Museum". history.nih.gov. Archived from the original on 26 December 2022. Retrieved 26 December 2022.
External links
- Yellow fever at Curlie
- Finlay CJ (2012). "The Mosquito Hypothetically Considered as the Transmitting Agent of Yellow Fever" (PDF). MEDICC Review. 14 (1): 56–9. PMID 34503309. Archived (PDF) from the original on 2020-12-09. Retrieved 2020-07-30.
- "U.S. Army Yellow Fever Commission." Archived 2017-04-26 at the Wayback Machine Claude Moore Health Sciences Library, University of Virginia
- "Yellow fever virus". NCBI Taxonomy Browser. 11089. Archived from the original on 2020-08-30. Retrieved 2020-07-30.
| Classification | |
|---|---|
| External resources |
- Pages with script errors
- CS1 maint: unrecognized language
- CS1 maint: uses authors parameter
- Webarchive template wayback links
- Articles with hatnote templates targeting a nonexistent page
- Articles containing Spanish-language text
- Articles with 'species' microformats
- Articles with medical app sidebar
- Articles with Curlie links
- Portal templates with all redlinked portals
- Yellow fever
- Biological weapons
- Epidemics
- Neglected tropical diseases
- RTT
- RTTID
- Tropical diseases
- Vaccine-preventable diseases