West Nile virus
| West Nile virus | |
|---|---|
| |
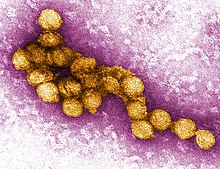
| A micrograph of the West Nile Virus, appearing in yellow | |
| Virus classification | |
| (unranked): | Virus |
| Realm: | Riboviria |
| Kingdom: | Orthornavirae |
| Phylum: | Kitrinoviricota |
| Class: | Flasuviricetes |
| Order: | Amarillovirales |
| Family: | Flaviviridae |
| Genus: | Flavivirus |
| Species: | West Nile virus
|
West Nile virus (WNV) is a single-stranded RNA virus that causes West Nile fever. It is a member of the family Flaviviridae, from the genus Flavivirus, which also contains the Zika virus, dengue virus, and yellow fever virus. The virus is primarily transmitted by mosquitoes, mostly species of Culex. The primary hosts of WNV are birds, so that the virus remains within a "bird–mosquito–bird" transmission cycle.The virus is genetically related to the Japanese encephalitis family of viruses.[1][2][3][4]
Humans and horses both exhibit disease symptoms from the virus, and symptoms rarely occur in other animals. Identification of the human disease was first made in 1937 in Uganda and in the latter half of the 20th century spread to many other parts of the world.[1][5]
Structure

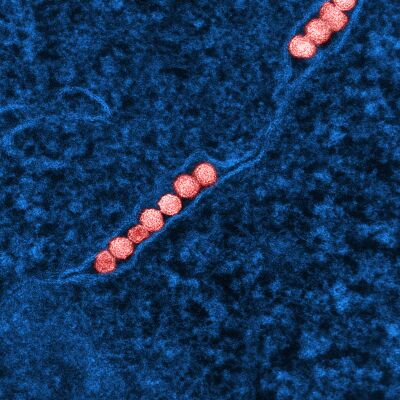
Like most other flaviviruses, WNV is an enveloped virus with icosahedral symmetry.[6] Electron microscope studies reveal a 45–50 nm virion covered with a relatively smooth protein shell; this structure is similar to the dengue fever virus, another Flavivirus.[6] The protein shell is made of two structural proteins: the glycoprotein E and the small membrane protein M.[7] Protein E has numerous functions including receptor binding, viral attachment, and entry into the cell through membrane fusion.[7]
The outer protein shell is covered by a host-derived lipid membrane, the viral envelope.[8] The flavivirus lipid membrane has been found to contain cholesterol and phosphatidylserine, but other elements of the membrane have yet to be identified.[9][10] The lipid membrane has many roles in viral infection, including acting as signaling molecules and enhancing entry into the cell.[11] Cholesterol, in particular, plays an integral part in WNV entering a host cell.[12] The two viral envelope proteins, E and M, are inserted into the membrane.[7]
The RNA genome is bound to capsid (C) proteins, which are 105 amino-acid residues long, to form the nucleocapsid. The capsid proteins are one of the first proteins created in an infected cell;[8] the capsid protein is a structural protein whose main purpose is to package RNA into the developing viruses.[13] The capsid has been found to prevent apoptosis by affecting the Akt pathway.[8]
Genome
WNV is a positive-sense, single-stranded RNA virus. Its genome is approximately 11,000 nucleotides long and is flanked by 5′ and 3′ non-coding stem loop structures.[14] The coding region of the genome codes for three structural proteins and seven nonstructural (NS) proteins, proteins that are not incorporated into the structure of new viruses. The WNV genome is first translated into a polyprotein and later cleaved by virus and host proteases into separate proteins (i.e. NS1, C, E).[15]

Structural proteins
Structural proteins (C, prM/M, E) are capsid, precursor membrane proteins, and envelope proteins, respectively, the structural proteins are located at the 5′ end of the genome and are cleaved into mature proteins by both host and viral proteases.[14][6]
| Structural Protein | Function |
|---|---|
| C | Capsid protein; encloses the RNA genome, packages RNA into immature virions.[13][18] |
| prM/M | Viruses with M protein are infectious: the presence of M protein allows for the activation of proteins involved in viral entry into the cell. prM (precursor membrane) protein is present on immature virions, by further cleavage by furin to M protein, the virions become infectious.[19] |
| E | A glycoprotein that forms the viral envelope, binds to receptors on the host cell surface in order to enter the cell.[20] |
Nonstructural proteins
Nonstructural proteins consist of NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5, these proteins mainly assist with viral replication or act as proteases, the nonstructural proteins are located near the 3′ end of the genome.[18][21][22]
| Nonstructural Protein | Function |
|---|---|
| NS1 | NS1 is a cofactor for viral replication, specifically for regulation of the replication complex.[21] |
| NS2A | NS2A has a variety of functions: it is involved in viral replication, virion assembly, and inducing host cell death.[23] |
| NS2B | A cofactor for NS3 and together forms the NS2B-NS3 protease complex, contains transmembrane domains which bind the protease to intracellular membranes.[18][24] |
| NS3 | A serine protease that is responsible for cleaving the polyprotein to produce mature proteins; it also acts as a helicase.[14] |
| NS4A | NS4A is a cofactor for viral replication, specifically regulates the activity of the NS3 helicase.[25] |
| NS4B | Inhibits interferon signaling.[26] |
| NS5 | The largest and most conserved protein of WNV, NS5 acts as a methyltransferase and a RNA polymerase, though it lacks proofreading properties.[18][27] |
Life cycle
Once WNV has successfully entered the bloodstream of a host animal, the envelope protein, E, binds to attachment factors called glycosaminoglycans on the host cell.[20] These attachment factors aid entry into the cell, however, binding to primary receptors is also necessary.[28] Primary receptors include DC-SIGN, DC-SIGN-R, and the integrin αvβ3.[29] By binding to these primary receptors, WNV enters the cell through clathrin-mediated endocytosis. As a result of endocytosis, WNV enters the cell within an endosome.[30][31]
The acidity of the endosome catalyzes the fusion of the endosomal and viral membranes, allowing the genome to be released into the cytoplasm.[32] Translation of the positive-sense single-stranded RNA occurs at the endoplasmic reticulum; the RNA is translated into a polyprotein which is then cleaved by both host and viral proteases NS2B-NS3 to produce mature proteins.[33]
In order to replicate its genome, NS5, a RNA polymerase, forms a replication complex with other nonstructural proteins to produce an intermediary negative-sense single-stranded RNA; the negative-sense strand serves as a template for synthesis of the final positive-sense RNA.[28] Once the positive-sense RNA has been synthesized, the capsid protein, C, encloses the RNA strands into immature virions.[29] The rest of the virus is assembled along the endoplasmic reticulum and through the Golgi apparatus, and results in non-infectious immature virions.[33] The E protein is then glycosylated and prM is cleaved by furin, a host cell protease, into the M protein, thereby producing an infectious mature virion. The mature viruses are then secreted out of the cell.[34][14][33]
Phylogeny

WNV is one of the Japanese encephalitis antigenic serocomplex of viruses, together with Japanese encephalitis virus, Murray Valley encephalitis virus, Saint Louis encephalitis virus and some other flaviviruses.[36] Studies of phylogenetic lineages have determined that WNV emerged as a distinct virus around 1000 years ago.[37] This initial virus developed into two distinct lineages. Lineage 1 and its multiple profiles is the source of the epidemic transmission in Africa and throughout the world. Lineage 2 was considered an African zoonosis. However, in 2008, lineage 2, previously only seen in horses in sub-Saharan Africa and Madagascar, began to appear in horses in Europe, where the first known outbreak affected 18 animals in Hungary.[38] Lineage 1 West Nile virus was detected in South Africa in 2010 in a mare and her aborted fetus; previously, only lineage 2 West Nile virus had been detected in horses and humans in South Africa.[39] Kunjin virus is a subtype of West Nile virus endemic to Oceania. A 2007 fatal case in a killer whale in Texas broadened the known host range of West Nile virus to include cetaceans.[40]
Since the first North American cases in 1999, the virus has been reported throughout the United States, Canada, Mexico, the Caribbean, and Central America. There have been human cases and equine cases, and many birds are infected. The Barbary macaque, Macaca sylvanus, was the first nonhuman primate to contract WNV.[41] Both the American and Israeli strains are marked by high mortality rates in infected avian populations; the presence of dead birds can be an early indicator of the arrival of the virus.[42]
Host range and transmission

The natural hosts for WNV are birds and mosquitoes.[4] Over 300 different species of bird have been shown to be infected with the virus.[43][44] Some birds, including the American crow (Corvus brachyrhynchos), blue jay (Cyanocitta cristata) and greater sage-grouse (Centrocercus urophasianus), are killed by the infection, but others survive.[45][46] The American robin (Turdus migratorius) and house sparrow (Passer domesticus) are thought to be among the most important reservoir species in N. American and European cities.[47][48] Brown thrashers (Toxostoma rufum), gray catbirds (Dumetella carolinensis), northern cardinals (Cardinalis cardinalis), northern mockingbirds (Mimus polyglottos), wood thrushes (Hylocichla mustelina) and the dove family are among the other common N. American birds in which high levels of antibodies against WNV have been found.[45]
WNV has been demonstrated in a large number of mosquito species, but the most significant for viral transmission are Culex species that feed on birds, including Culex pipiens, C. restuans, C. salinarius, C. quinquefasciatus, C. nigripalpus, C. erraticus and C. tarsalis.[45] Experimental infection has also been demonstrated with soft tick vectors, but is unlikely to be important in natural transmission.[45][49]
WNV has a broad host range, and is also known to be able to infect at least 30 mammalian species, including humans, some non-human primates,[50] horses, dogs and cats.[43][47][51] Some infected humans and horses experience disease but dogs and cats rarely show symptoms.[43] Reptiles and amphibians can also be infected, including some species of crocodiles, alligators, snakes, lizards and frogs.[51][52][53][54] Mammals are considered incidental or dead-end hosts for the virus: they do not usually develop a high enough level of virus in the blood (viremia) to infect another mosquito feeding on them and carry on the transmission cycle; some birds are also dead-end hosts.[45]
In the normal rural or enzootic transmission cycle, the virus alternates between the bird reservoir and the mosquito vector. It can also be transmitted between birds via direct contact, by eating an infected bird carcass or by drinking infected water.[48] Vertical transmission between female and offspring is possible in mosquitoes, and might potentially be important in overwintering.[55][56] In the urban or spillover cycle, infected mosquitoes that have fed on infected birds transmit the virus to humans. This requires mosquito species that bite both birds and humans, which are termed bridge vectors.[48][57] The virus can also rarely be spread through blood transfusions, organ transplants, or from mother to baby during pregnancy, delivery, or breastfeeding.[57] Unlike in birds, it does not otherwise spread directly between people.[4]
Disease
Humans
West Nile fever is an infection by the West Nile virus, which is typically spread by mosquitoes.[57] In about 80% of infections people have few or no symptoms.[58] About 20% of people develop a fever, headache, vomiting, or a rash.[57] In less than 1% of people, encephalitis or meningitis occurs, with associated neck stiffness, confusion, or seizures.[57] Recovery may take weeks to months.[57] The risk of death among those in whom the nervous system is affected is about 10%.[57]
West Nile virus (WNV) is usually spread by mosquitoes that become infected when they feed on infected birds, which often carry the disease.[57] Rarely the virus is spread through blood transfusions, organ transplants, or from mother to baby during pregnancy, delivery, or breastfeeding,[57] but it otherwise does not spread directly between people.[4] Risks for severe disease include being over 60 years old and having other health problems.[57] Diagnosis is typically based on symptoms and blood tests.[57]
There is no human vaccine.[57] The best way to reduce the risk of infection is to avoid mosquito bites.[57] Mosquito populations may be reduced by eliminating standing pools of water, such as in old tires, buckets, gutters, and swimming pools.[57] When mosquitoes cannot be avoided, mosquito repellent, window screens, and mosquito nets reduce the likelihood of being bitten.[57][4] There is no specific treatment for the disease; pain medications may reduce symptoms.[57]
The virus was discovered in Uganda in 1937, and was first detected in North America in 1999.[57][4] WNV has occurred in Europe, Africa, Asia, Australia, and North America.[57] In the United States thousands of cases are reported a year, with most occurring in August and September.[59] It can occur in outbreaks of disease.[4] Severe disease may also occur in horses, for which a vaccine is available.[4] A surveillance system in birds is useful for early detection of a potential human outbreak.[4]
-
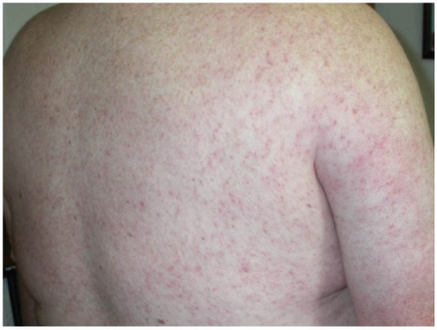
Diffuse maculopapular rash associated with West Nile virus infection.
-
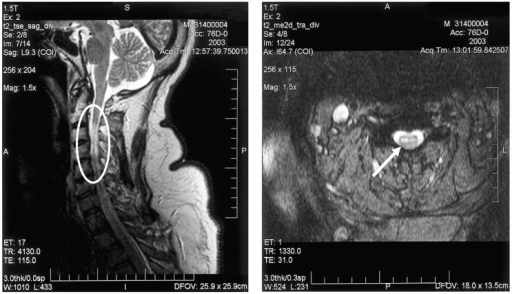
a,b)MRI of cervical spinal cord of individual with bilateral upper extremity paralysis and respiratory failure from West Nile poliomyelitis
Horses
Severe disease may also occur in horses.[4] Several vaccines for these animals are now available.[60][4] Before the availability of veterinary vaccines, around 40% of horses infected in North America died.[45]
Epidemiology
According to the Center for Disease Control, infection with West Nile Virus is seasonal in temperate zones. Climates that are temperate, such as those in the United States and Europe, see peak season from July to October. Peak season changes depending on geographic region and warmer and humid climates can see longer peak seasons.[61] [62]All ages are equally likely to be infected but there is a higher amount of death and neuroinvasive West Nile Virus in people 60–89 years old, hence people of older age are more likely to have adverse effects.[1][63]
There are several modes of transmission, but the most common cause of infection in humans is by being bitten by an infected mosquito. Other modes of transmission include blood transfusion, organ transplantation, breast-feeding, transplacental transmission, and laboratory acquisition. These alternative modes of transmission are extremely rare.[64]
-
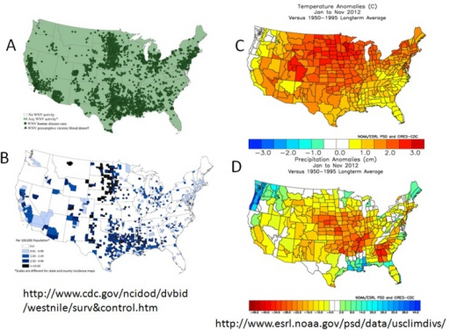
a-d) West Nile virus activity and climate analomies in the USA during 2012
-
Global distribution of West Nile Virus from the CDC
Prevention
Prevention efforts against WNV mainly focus on preventing human contact with and being bitten by infected mosquitoes. This is twofold, first by personal protective actions and second by mosquito-control actions. When a person is in an area that has WNV, it is important to avoid outdoor activity, and if they go outside they should use a mosquito repellent with DEET.[64] A person can also wear clothing that covers more skin, such as long sleeves and pants. Mosquito control can be done at the community level and include surveillance programs and control programs including pesticides and reducing mosquito habitats. This includes draining standing water. Surveillance systems in birds is particularly useful.[65] If dead birds are found in a neighborhood, the event should be reported to local authorities. This may help health departments do surveillance and determine if the birds are infected with West Nile Virus.[66]
Despite the commercial availability of four veterinary vaccines for horses, no human vaccine has progressed beyond phase II clinical trials.[60][57][67] Efforts have been made to produce a vaccine for human use and several candidates have been produced but none are licensed to use.[64][67] The best method to reduce the risk of infections is avoiding mosquito bites.[57] This may be done by eliminating standing pools of water, such as in old tires, buckets, gutters, and swimming pools.[57] Mosquito repellent, window screens, mosquito nets, and avoiding areas where mosquitoes occur may also be useful.[57][4]
Climate change

Like other tropical diseases which are expected to have increased spread due to climate change, there is concern that changing weather conditions will increase West Nile Virus spread. Climate change will affect disease rates, ranges, seasonality and affects the distribution of West Nile Virus.[68]
Projected changes in flood frequency and severity can bring new challenges in flood risk management, allowing for increased mosquito populations in urban areas.[69] Weather conditions affected by climate change including temperature, precipitation and wind may affect the survival and reproduction rates of mosquitoes, suitable habitats, distribution, and abundance. Ambient temperatures drive mosquito replication rates and transmission of WNV by affecting the peak season of mosquitoes and geographic variations. For example, increased temperatures can affect the rate of virus replication, speed up the virus evolution rate, and viral transmission efficiency. Furthermore, higher winter temperatures and warmer spring may lead to larger summer mosquito populations, increasing the risk for WNV. Similarly, rainfall may also drive mosquito replication rates and affect the seasonality and geographic variations of the virus. Studies show an association between heavy precipitation and higher incidence of reported WNV. Likewise, wind is another environmental factor that serves as a dispersal mechanism for mosquitoes.[68]
Mosquitoes have extremely wide environmental tolerances and a nearly ubiquitous geographical distribution, being present on all major land masses except Antarctica and Iceland. Nevertheless, changes in climate and land use on ecological timescales can variously expand or fragment their distribution patterns, raising consequent concerns for human health.[70]
References
- ↑ 1.0 1.1 1.2 Clark, Michael B.; Schaefer, Timothy J. (2023). "West Nile Virus". StatPearls. StatPearls Publishing. Archived from the original on 10 January 2021. Retrieved 20 April 2023.
- ↑ Mackenzie, John S; Gubler, Duane J; Petersen, Lyle R (2004). "Emerging flaviviruses: the spread and resurgence of Japanese encephalitis, West Nile and dengue viruses". Nature Medicine. 10 (12s): S98–S109. doi:10.1038/nm1144. PMID 15577938. S2CID 9987454.
- ↑ Karim, Shazeed-Ul; Bai, Fengwei (2023). "Introduction to West Nile Virus". Methods in Molecular Biology (Clifton, N.J.). 2585: 1–7. doi:10.1007/978-1-0716-2760-0_1. ISSN 1940-6029. Archived from the original on 4 November 2022. Retrieved 25 April 2023.
- ↑ 4.00 4.01 4.02 4.03 4.04 4.05 4.06 4.07 4.08 4.09 4.10 4.11 "West Nile virus". World Health Organization. July 2011. Archived from the original on 18 October 2017. Retrieved 28 October 2017.
- ↑ Hayes, C. G. (December 2001). "West Nile virus: Uganda, 1937, to New York City, 1999". Annals of the New York Academy of Sciences. 951: 25–37. doi:10.1111/j.1749-6632.2001.tb02682.x. ISSN 0077-8923. Archived from the original on 15 April 2023. Retrieved 25 April 2023.
- ↑ 6.0 6.1 6.2 Mukhopadhyay, Suchetana; Kim, Bong-Suk; Chipman, Paul R.; Rossmann, Michael G.; Kuhn, Richard J. (2003-10-10). "Structure of West Nile Virus". Science. 302 (5643): 248. doi:10.1126/science.1089316. ISSN 0036-8075. PMID 14551429. S2CID 23555900. Archived from the original on 2023-04-14. Retrieved 2023-03-15.
- ↑ 7.0 7.1 7.2 Kanai, Ryuta; Kar, Kalipada; Anthony, Karen; Gould, L. Hannah; Ledizet, Michel; Fikrig, Erol; Marasco, Wayne A.; Koski, Raymond A.; Modis, Yorgo (2006-11-01). "Crystal Structure of West Nile Virus Envelope Glycoprotein Reveals Viral Surface Epitopes". Journal of Virology. 80 (22): 11000–11008. doi:10.1128/jvi.01735-06. ISSN 0022-538X. PMC 1642136. PMID 16943291.
- ↑ 8.0 8.1 8.2 Urbanowski, Matt D.; Hobman, Tom C. (2013-01-15). "The West Nile Virus Capsid Protein Blocks Apoptosis through a Phosphatidylinositol 3-Kinase-Dependent Mechanism". Journal of Virology. 87 (2): 872–881. doi:10.1128/jvi.02030-12. ISSN 0022-538X. PMC 3554064. PMID 23115297.
- ↑ Meertens, Laurent; Carnec, Xavier; Lecoin, Manuel Perera; Ramdasi, Rasika; Guivel-Benhassine, Florence; Lew, Erin; Lemke, Greg; Schwartz, Olivier; Amara, Ali (2012). "The TIM and TAM Families of Phosphatidylserine Receptors Mediate Dengue Virus Entry". Cell Host & Microbe. 12 (4): 544–557. doi:10.1016/j.chom.2012.08.009. PMC 3572209. PMID 23084921.
- ↑ Carro, Ana C.; Damonte, Elsa B. (2013). "Requirement of cholesterol in the viral envelope for dengue virus infection". Virus Research. 174 (1–2): 78–87. doi:10.1016/j.virusres.2013.03.005. PMID 23517753.
- ↑ Martín-Acebes, Miguel A.; Merino-Ramos, Teresa; Blázquez, Ana-Belén; Casas, Josefina; Escribano-Romero, Estela; Sobrino, Francisco; Saiz, Juan-Carlos (2014-10-15). "The Composition of West Nile Virus Lipid Envelope Unveils a Role of Sphingolipid Metabolism in Flavivirus Biogenesis". Journal of Virology. 88 (20): 12041–12054. doi:10.1128/jvi.02061-14. ISSN 0022-538X. PMC 4178726. PMID 25122799.
- ↑ Medigeshi, Guruprasad R.; Hirsch, Alec J.; Streblow, Daniel N.; Nikolich-Zugich, Janko; Nelson, Jay A. (2008-06-01). "West Nile Virus Entry Requires Cholesterol-Rich Membrane Microdomains and Is Independent of αvβ3 Integrin". Journal of Virology. 82 (11): 5212–5219. doi:10.1128/jvi.00008-08. ISSN 0022-538X. PMC 2395215. PMID 18385233.
- ↑ 13.0 13.1 Hunt, Tracey A.; Urbanowski, Matthew D.; Kakani, Kishore; Law, Lok-Man J.; Brinton, Margo A.; Hobman, Tom C. (2007-11-01). "Interactions between the West Nile virus capsid protein and the host cell-encoded phosphatase inhibitor, I2PP2A". Cellular Microbiology. 9 (11): 2756–2766. doi:10.1111/j.1462-5822.2007.01046.x. ISSN 1462-5822. PMID 17868381.
- ↑ 14.0 14.1 14.2 14.3 Colpitts, Tonya M.; Conway, Michael J.; Montgomery, Ruth R.; Fikrig, Erol (2012-10-01). "West Nile Virus: Biology, Transmission, and Human Infection". Clinical Microbiology Reviews. 25 (4): 635–648. doi:10.1128/cmr.00045-12. ISSN 0893-8512. PMC 3485754. PMID 23034323.
- ↑ Chung, Kyung Min; Liszewski, M. Kathryn; Nybakken, Grant; Davis, Alan E.; Townsend, R. Reid; Fremont, Daved H.; Atkinson, John P.; Diamond, Michael S. (2006-12-12). "West Nile virus nonstructural protein NS1 inhibits complement activation by binding the regulatory protein factor H". Proceedings of the National Academy of Sciences. 103 (50): 19111–19116. doi:10.1073/pnas.0605668103. ISSN 0027-8424. PMC 1664712. PMID 17132743.
- ↑ Guzman, Maria G.; Halstead, Scott B.; Artsob, Harvey; Buchy, Philippe; Farrar, Jeremy; Gubler, Duane J.; Hunsperger, Elizabeth; Kroeger, Axel; Margolis, Harold S. (2010-12-01). "Dengue: a continuing global threat" (PDF). Nature Reviews Microbiology. 8 (12): S7–S16. doi:10.1038/nrmicro2460. ISSN 1740-1534. PMC 4333201. PMID 21079655.
- ↑ "Dengue Viruses". www.nature.com. Retrieved 2017-12-19.
- ↑ 18.0 18.1 18.2 18.3 Londono-Renteria, Berlin; Colpitts, Tonya M. (2016). A Brief Review of West Nile Virus Biology. Methods in Molecular Biology. Vol. 1435. pp. 1–13. doi:10.1007/978-1-4939-3670-0_1. ISBN 978-1-4939-3668-7. ISSN 1940-6029. PMID 27188545.
- ↑ Moesker, Bastiaan; Rodenhuis-Zybert, Izabela A.; Meijerhof, Tjarko; Wilschut, Jan; Smit, Jolanda M. (2010). "Characterization of the functional requirements of West Nile virus membrane fusion". Journal of General Virology. 91 (2): 389–393. doi:10.1099/vir.0.015255-0. PMID 19828760.
- ↑ 20.0 20.1 Perera-Lecoin, Manuel; Meertens, Laurent; Carnec, Xavier; Amara, Ali (2013-12-30). "Flavivirus Entry Receptors: An Update". Viruses. 6 (1): 69–88. doi:10.3390/v6010069. PMC 3917432. PMID 24381034.
- ↑ 21.0 21.1 Youn, Soonjeon; Ambrose, Rebecca L.; Mackenzie, Jason M.; Diamond, Michael S. (18 November 2013). "Non-structural protein-1 is required for West Nile virus replication complex formation and viral RNA synthesis". Virology Journal. 10 (1): 339. doi:10.1186/1743-422X-10-339. ISSN 1743-422X. Archived from the original on 24 February 2023. Retrieved 24 April 2023.
- ↑ Knyazhanskaya, Ekaterina; Morais, Marc C.; Choi, Kyung H. (2021). "Flavivirus enzymes and their inhibitors". The Enzymes. 49: 265–303. doi:10.1016/bs.enz.2021.07.006. ISSN 0423-2607. Archived from the original on 26 March 2023. Retrieved 26 April 2023.
- ↑ Melian, Ezequiel Balmori; Edmonds, Judith H.; Nagasaki, Tomoko Kim; Hinzman, Edward; Floden, Nadia; Khromykh, Alexander A. (2013). "West Nile virus NS2A protein facilitates virus-induced apoptosis independently of interferon response". Journal of General Virology. 94 (2): 308–313. doi:10.1099/vir.0.047076-0. PMC 3709616. PMID 23114626.
- ↑ Bai, Fengwei (4 November 2022). West Nile Virus: Methods and Protocols. Springer Nature. p. 2. ISBN 978-1-0716-2760-0. Archived from the original on 26 April 2023. Retrieved 26 April 2023.
- ↑ Shiryaev, Sergey A.; Chernov, Andrei V.; Aleshin, Alexander E.; Shiryaeva, Tatiana N.; Strongin, Alex Y. (2009). "NS4A regulates the ATPase activity of the NS3 helicase: a novel cofactor role of the non-structural protein NS4A from West Nile virus". Journal of General Virology. 90 (9): 2081–2085. doi:10.1099/vir.0.012864-0. PMC 2887571. PMID 19474250.
- ↑ Wicker, Jason A.; Whiteman, Melissa C.; Beasley, David W.C.; Davis, C. Todd; McGee, Charles E.; Lee, J. Ching; Higgs, Stephen; Kinney, Richard M.; Huang, Claire Y.-H. (2012). "Mutational analysis of the West Nile virus NS4B protein". Virology. 426 (1): 22–33. doi:10.1016/j.virol.2011.11.022. PMC 4583194. PMID 22314017.
- ↑ Davidson, Andrew D. (2009). Chapter 2 New Insights into Flavivirus Nonstructural Protein 5. Advances in Virus Research. Vol. 74. pp. 41–101. doi:10.1016/s0065-3527(09)74002-3. ISBN 9780123785879. PMID 19698895.
- ↑ 28.0 28.1 Brinton, Margo A. (2002-10-01). "The Molecular Biology of West Nile Virus: A New Invader of the Western Hemisphere". Annual Review of Microbiology. 56 (1): 371–402. doi:10.1146/annurev.micro.56.012302.160654. ISSN 0066-4227. PMID 12142476.
- ↑ 29.0 29.1 Samuel, Melanie A.; Diamond, Michael S. (2006-10-01). "Pathogenesis of West Nile Virus Infection: a Balance between Virulence, Innate and Adaptive Immunity, and Viral Evasion". Journal of Virology. 80 (19): 9349–9360. doi:10.1128/jvi.01122-06. ISSN 0022-538X. PMC 1617273. PMID 16973541.
- ↑ Vancini, Ricardo; Kramer, Laura D.; Ribeiro, Mariana; Hernandez, Raquel; Brown, Dennis (2013). "Flavivirus infection from mosquitoes in vitro reveals cell entry at the plasma membrane". Virology. 435 (2): 406–414. doi:10.1016/j.virol.2012.10.013. PMID 23099205.
- ↑ "Factsheet about West Nile virus infection". www.ecdc.europa.eu. 23 August 2010. Archived from the original on 4 April 2022. Retrieved 21 April 2023.
- ↑ Mukhopadhyay, Suchetana; Kuhn, Richard J.; Rossmann, Michael G. (2005). "A structural perspective of the flavivirus life cycle". Nature Reviews Microbiology. 3 (1): 13–22. doi:10.1038/nrmicro1067. PMID 15608696. S2CID 4150641.
- ↑ 33.0 33.1 33.2 Suthar, Mehul S.; Diamond, Michael S.; Gale, Michael Jr. (2013). "West Nile virus infection and immunity". Nature Reviews Microbiology. 11 (2): 115–128. doi:10.1038/nrmicro2950. PMID 23321534. S2CID 1013677.
- ↑ Saiz, Juan-Carlos; Martín-Acebes, Miguel A.; Blázquez, Ana B.; Escribano-Romero, Estela; Poderoso, Teresa; Jiménez de Oya, Nereida (31 December 2021). "Pathogenicity and virulence of West Nile virus revisited eight decades after its first isolation". Virulence. 12 (1): 1145–1173. doi:10.1080/21505594.2021.1908740. Retrieved 20 April 2023.
- ↑ Lanciotti RS, Ebel GD, Deubel V, et al. (June 2002). "Complete genome sequences and phylogenetic analysis of West Nile virus strains isolated from the United States, Europe, and the Middle East". Virology. 298 (1): 96–105. doi:10.1006/viro.2002.1449. PMID 12093177. S2CID 17275232.
- ↑ Lobigs M, Diamond MS (2012). "Feasibility of cross-protective vaccination against flaviviruses of the Japanese encephalitis serocomplex". Expert Rev Vaccines. 11 (2): 177–87. doi:10.1586/erv.11.180. PMC 3337329. PMID 22309667.
- ↑ Galli M, Bernini F, Zehender G (July 2004). "Alexander the Great and West Nile virus encephalitis". Emerging Infect. Dis. 10 (7): 1330–2, author reply 1332–3. doi:10.3201/eid1007.040396. PMID 15338540.
- ↑ West, Christy (2010-02-08). "Different West Nile Virus Genetic Lineage Evolving?". The Horse. Archived from the original on 2010-02-17. Retrieved 2010-02-10. From statements by Orsolya Kutasi, DVM, of the Szent Istvan University, Hungary at the 2009 American Association of Equine Practitioners Convention, December 5–9, 2009.
- ↑ Venter M, Human S, van Niekerk S, Williams J, van Eeden C, Freeman F (August 2011). "Fatal neurologic disease and abortion in mare infected with lineage 1 West Nile virus, South Africa". Emerging Infect. Dis. 17 (8): 1534–6. doi:10.3201/eid1708.101794. PMC 3381566. PMID 21801644.
- ↑ St Leger J, Wu G, Anderson M, Dalton L, Nilson E, Wang D (2011). "West Nile virus infection in killer whale, Texas, USA, 2007". Emerging Infect. Dis. 17 (8): 1531–3. doi:10.3201/eid1708.101979. PMC 3381582. PMID 21801643.
- ↑ Hogan, C. Michael (2008). Barbary Macaque: Macaca sylvanus, GlobalTwitcher.com Archived 2009-08-31 at the Wayback Machine
- ↑ Bakonyi, Tamas; Ivanics,, Eva; Erdélyi, Karoly; Ursu,, Kristina (2006). "Lineage 1 and 2 Strains of Encephalitic West Nile Virus, Central Europe". Emerging infectious diseases. 12 (4). Archived from the original on 23 April 2023. Retrieved 23 April 2023.
{{cite journal}}: CS1 maint: extra punctuation (link) - ↑ 43.0 43.1 43.2 "Vertebrate Ecology". West Nile Virus. Division of Vector-Borne Diseases, CDC. 30 April 2009. Archived from the original on 1 March 2013.
- ↑ "Species of dead birds in which West Nile virus has been detected, United States, 1999–2016" (PDF). CDC. Archived (PDF) from the original on 30 June 2018. Retrieved 28 March 2019.
- ↑ 45.0 45.1 45.2 45.3 45.4 45.5 Kilpatrick, AM; SL LaDeau; PP Marra (2007). "Ecology of West Nile virus transmission and its impact on birds in the western hemisphere" (PDF). The Auk. 124 (4): 1121–36. doi:10.1642/0004-8038(2007)124[1121:EOWNVT]2.0.CO;2. S2CID 13796761. Archived (PDF) from the original on 2023-03-15. Retrieved 2023-03-15.
- ↑ Kaci K. VanDalen; Jeffrey S. Hall; Larry Clark; Robert G. McLean; Cynthia Smeraski (2013). "West Nile Virus Infection in American Robins: New Insights on Dose Response". PLOS One. 8 (7): e68537. Bibcode:2013PLoSO...868537V. doi:10.1371/journal.pone.0068537. PMC 3699668. PMID 23844218.
- ↑ 47.0 47.1 Kilpatrick, AM; P Daszak; MJ Jones; PP Marra; LD Kramer (2006). "Host heterogeneity dominates West Nile virus transmission". Proceedings of the Royal Society B: Biological Sciences. 273 (1599): 2327–2333. doi:10.1098/rspb.2006.3575. PMC 1636093. PMID 16928635.
- ↑ 48.0 48.1 48.2 Virginia Gamino; Ursula Höfle (2013). "Pathology and tissue tropism of natural West Nile virus infection in birds: a review". Veterinary Research. 44 (1): 39. doi:10.1186/1297-9716-44-39. PMC 3686667. PMID 23731695.
- ↑ Lawrie, Charles; Uzcátegui, Nathalie; Gould, Ernest; Nuttall, Patricia (April 2004). "Ixodid and Argasid Tick Species and West Nile Virus". Emerging Infectious Diseases. 10 (4): 653–657. doi:10.3201/eid1004.030517. PMC 3323096. PMID 15200855.
- ↑ Marion S. Ratterree; Amelia P.A. Travassos da Rosa; Rudolf P. Bohm Jr.; et al. (2003). "West Nile Virus Infection in Nonhuman Primate Breeding Colony, Concurrent with Human Epidemic, Southern Louisiana" (PDF). Emerging Infectious Diseases. 9 (11): 1388–94. doi:10.3201/eid0911.030226. PMID 14718080. Archived (PDF) from the original on 2023-03-14. Retrieved 2023-03-15.
- ↑ 51.0 51.1 Peter P. Marra; Sean Griffing; Carolee Caffrey; et al. (2004). "West Nile Virus and Wildlife". BioScience. 54 (5): 393–402. doi:10.1641/0006-3568(2004)054[0393:WNVAW]2.0.CO;2.
- ↑ Amir Steinman; Caroline Banet-Noach; Shlomit Tal; et al. (2003). "West Nile Virus Infection in Crocodiles". Emerging Infectious Diseases. 9 (7): 887–89. doi:10.3201/eid0907.020816. PMC 3023443. PMID 12899140.
- ↑ C. R. Dahlina; D. F. Hughes; W. E. Meshaka Jr.; C. Coleman; J. D. Henning (2016). "Wild snakes harbor West Nile virus". One Health. 2: 136–38. doi:10.1016/j.onehlt.2016.09.003. PMC 5441359. PMID 28616487.
- ↑ Ellen Ariel (2011). "Viruses in reptiles". Veterinary Research. 42 (1): 100. doi:10.1186/1297-9716-42-100. PMC 3188478. PMID 21933449.
- ↑ Goddard LB, Roth AE, Reisen WK, Scott TW (November 2003). "Vertical transmission of West Nile Virus by three California Culex (Diptera: Culicidae) species". J. Med. Entomol. 40 (6): 743–6. doi:10.1603/0022-2585-40.6.743. PMID 14765647.
- ↑ Bugbee, LM; Forte LR (September 2004). "The discovery of West Nile virus in overwintering Culex pipiens (Diptera: Culicidae) mosquitoes in Lehigh County, Pennsylvania". Journal of the American Mosquito Control Association. 20 (3): 326–7. PMID 15532939.
- ↑ 57.00 57.01 57.02 57.03 57.04 57.05 57.06 57.07 57.08 57.09 57.10 57.11 57.12 57.13 57.14 57.15 57.16 57.17 57.18 57.19 57.20 57.21 "General Questions About West Nile Virus". www.cdc.gov. 19 October 2017. Archived from the original on 26 October 2017. Retrieved 26 October 2017.
- ↑ "Symptoms, Diagnosis, & Treatment". www.cdc.gov. 15 January 2019. Archived from the original on 26 October 2017. Retrieved 15 January 2019.
- ↑ "Final Cumulative Maps and Data | West Nile Virus | CDC". www.cdc.gov. 24 October 2017. Archived from the original on 27 October 2017. Retrieved 28 October 2017.
- ↑ 60.0 60.1 Seino, K. K.; Long, M. T.; Gibbs, E. P. J.; Bowen, R. A.; Beachboard, S. E.; Humphrey, P. P.; Dixon, M. A.; Bourgeois, M. A. (2007-11-01). "Comparative Efficacies of Three Commercially Available Vaccines against West Nile Virus (WNV) in a Short-Duration Challenge Trial Involving an Equine WNV Encephalitis Model". Clinical and Vaccine Immunology. 14 (11): 1465–1471. doi:10.1128/CVI.00249-07. ISSN 1556-6811. PMC 2168174. PMID 17687109.
- ↑ Hayes, Edward B.; Komar, Nicholas; Nasci, Roger S.; Montgomery, Susan P.; O'Leary, Daniel R.; Campbell, Grant L. (August 2005). "Epidemiology and Transmission Dynamics of West Nile Virus Disease". Emerging Infectious Diseases. 11 (8): 1167–1173. doi:10.3201/eid1108.050289a. ISSN 1080-6040. PMC 3320478. PMID 16102302.
- ↑ "West Nile Virus | NIH: National Institute of Allergy and Infectious Diseases". www.niaid.nih.gov. Archived from the original on 3 April 2023. Retrieved 20 April 2023.
- ↑ Levi, Marilyn E. (15 November 2013). "West Nile Virus Infection in the Immunocompromised Patient". Current Infectious Disease Reports. doi:10.1007/s11908-013-0367-8. ISSN 1523-3847. Archived from the original on 15 June 2022. Retrieved 24 April 2023.
- ↑ 64.0 64.1 64.2 Sampathkumar, Priya (September 2003). "West Nile Virus: Epidemiology, Clinical Presentation, Diagnosis, and Prevention". Mayo Clinic Proceedings. 78 (9): 1137–1144. doi:10.4065/78.9.1137. ISSN 0025-6196. PMC 7125680. PMID 12962168.
- ↑ "West Nile Virus Activity—United States, October 17-23, 2001". JAMA. 286 (18): 2232. 2001-11-14. doi:10.1001/jama.286.18.2232-jwr10138-2-1. ISSN 0098-7484.
- ↑ McCormick, Sabrina; Whitney, Kristoffer (2012-12-20). "The making of public health emergencies: West Nile virus in New York City". Sociology of Health & Illness. 35 (2): 268–279. doi:10.1111/1467-9566.12002. ISSN 0141-9889. PMID 23278188.
- ↑ 67.0 67.1 Kaiser, Jaclyn A.; Barrett, Alan D.T. (2019-09-05). "Twenty Years of Progress Toward West Nile Virus Vaccine Development". Viruses. 11 (9): 823. doi:10.3390/v11090823. ISSN 1999-4915. PMC 6784102. PMID 31491885.
- ↑ 68.0 68.1 Paz, Shlomit (2015-04-05). "Climate change impacts on West Nile virus transmission in a global context". Philosophical Transactions of the Royal Society B: Biological Sciences. 370 (1665): 20130561. doi:10.1098/rstb.2013.0561. ISSN 0962-8436. PMC 4342965. PMID 25688020.
- ↑ Appendix 6: Topics for Consideration in Future Assessments. Climate Change Impacts in the United States: The Third National Climate Assessment. National Climate Assessment (Report). U.S. Global Change Research Program. 2014. doi:10.7930/j06h4fbf.
- ↑ Andersen, Louise K.; Davis, Mark D. P. (2016-10-01). "Climate change and the epidemiology of selected tick-borne and mosquito-borne diseases: update from the International Society of Dermatology Climate Change Task Force". International Journal of Dermatology. 56 (3): 252–259. doi:10.1111/ijd.13438. ISSN 0011-9059. PMID 27696381. S2CID 23187115.
Further reading
- Species Profile - West Nile Virus Archived 2019-08-13 at the Wayback Machine, National Invasive Species Information Center, United States National Agricultural Library. Lists general information and resources for West Nile Virus