Borna disease virus
| Mammalian 1 orthobornavirus | |
|---|---|
| Virus classification | |
| (unranked): | Virus |
| Realm: | Riboviria |
| Kingdom: | Orthornavirae |
| Phylum: | Negarnaviricota |
| Class: | Monjiviricetes |
| Order: | Mononegavirales |
| Family: | Bornaviridae |
| Genus: | Orthobornavirus |
| Species: | Mammalian 1 orthobornavirus
|
| Borna disease virus 1 G protein | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| Symbol | BDV_G | ||||||||
| Pfam | PF06208 | ||||||||
| InterPro | IPR009344 | ||||||||
| |||||||||
| Borna disease virus 1 P10 protein | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| Symbol | BDV_P10 | ||||||||
| Pfam | PF06515 | ||||||||
| InterPro | IPR009485 | ||||||||
| |||||||||
| Borna disease virus 1 P40 protein | |||||||||
|---|---|---|---|---|---|---|---|---|---|
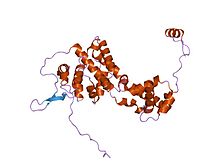 crystal structure of the borna disease virus 1 nucleoprotein | |||||||||
| Identifiers | |||||||||
| Symbol | BDV_P40 | ||||||||
| Pfam | PF06407 | ||||||||
| InterPro | IPR009441 | ||||||||
| SCOP2 | 1n93 / SCOPe / SUPFAM | ||||||||
| |||||||||
| Borna disease virus 1 P24 protein | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| Symbol | BDV_P24 | ||||||||
| Pfam | PF06595 | ||||||||
| InterPro | IPR009517 | ||||||||
| |||||||||
The Borna disease viruses 1 and 2 (BoDV-1 and BoDV-2) are members of the species Mammalian 1 orthobornavirus and cause Borna disease in mammals.
Virology
Genome
BoDV-1/2 have the smallest genome (8.9 kilobases) of any Mononegavirales member and are unique within that order in their ability to replicate within the host cell nucleus.
BoDV-1 was isolated from a diseased horse in the 1970s, but the virus particles were difficult to characterise. Nonetheless, the virus' genome has been characterised. It is a linear negative-sense single stranded RNA virus in the order Mononegavirales.
Several of the proteins encoded by the BoDV-1 genome have been characterised. The G glycoprotein is important for viral entry into the host cell.[1][2]
It has been suggested that the p10, or X, protein plays a role in viral RNA synthesis or ribonucleoprotein transport.[3]
The P40 nucleoprotein from BoDV-1 is multi-helical in structure and can be divided into two subdomains, each of which has an alpha-bundle topology.[4] The nucleoprotein assembles into a planar homotetramer, with the RNA genome either wrapping around the outside of the tetramer or possibly fitting within the charged central channel of the tetramer .
P24 (phosphoprotein 24) is an essential component of the RNA polymerase transcription and replication complex. P24 is encoded by open reading frame II (ORF-II) and undergoes high rates of mutation in humans. It [binds amphoterin-HMGB1, a multifunctional protein, directly and may cause deleterious effects in cellular functions by interference with HMGB1.[5] Horse and human P24 have no species-specific amino acid residues, suggesting that the two viruses are related.[6][7] Numerous interactions of the immune system with the central nervous system have been described. Mood and psychotic disorders, such as severe depression and schizophrenia, are both heterogeneous disorders regarding clinical symptomatology, the acuity of symptoms, the clinical course and the treatment response.[8] BoDV-1 p24 RNA has been detected in the peripheral blood mononuclear cells (PBMCs) of psychiatric patients with such conditions.[7] Some studies find a significant difference in the prevalence of BDV p24 RNA in patients with mood disorders and schizophrenia,[9] whilst others find no difference between patients and control groups.[7] Consequently, debate about the role of BDV in psychiatric diseases remains alive.
Replication
Bornaviruses enter host cells by endocytosis. The viral genome and associated viral proteins is released into the cytoplasm following fusion of the viral envelope and the endosome membrane.[10] Replication of bornaviruses occurs inside the nucleus. These are the only animal viruses within the order Mononegavirales to do this. Many plant rhabdoviruses replicate in the nucleus.
Bornaviruses have negative sense RNA genomes[11] The negative sense RNA is copied to make a positive sense RNA template. This template is then used to synthesise many copies of the negative sense RNA genome. This is like making copies of a mold, and then using these molds to make many more viruses.
Endogenous provirus
Endogenous viral elements homologous to the nucleoprotein gene of BoDV-1 have been shown to exist in the genomes of several mammalian species, including humans and non-human primates.[12]
Evolution
A Bayesian analysis of Borna disease virus 1 suggests that the current strains diversified ~300 years ago and that avian-host bornaviruses evolved considerably earlier than this.[13] The ancestral virus seems likely to have been a high AT content virus.
History
Borna disease was first described in 1885 as "heated head disease" of cavalry horses in 1885 in the town of Borna, Germany.[14]
References
- ^ Schneider PA, Hatalski CG, Lewis AJ, Lipkin WI (January 1997). "Biochemical and functional analysis of the Borna disease virus G protein". Journal of Virology. 71 (1): 331–6. doi:10.1128/JVI.71.1.331-336.1997. PMC 191055. PMID 8985354.
- ^ Perez M, Watanabe M, Whitt MA, de la Torre JC (August 2001). "N-terminal domain of Borna disease virus G (p56) protein is sufficient for virus receptor recognition and cell entry". Journal of Virology. 75 (15): 7078–85. doi:10.1128/JVI.75.15.7078-7085.2001. PMC 114436. PMID 11435588.
- ^ Wolff T, Pfleger R, Wehner T, Reinhardt J, Richt JA (April 2000). "A short leucine-rich sequence in the Borna disease virus p10 protein mediates association with the viral phospho- and nucleoproteins". The Journal of General Virology. 81 (Pt 4): 939–47. doi:10.1099/0022-1317-81-4-939. PMID 10725419.
- ^ Planz O, Stitz L (February 1999). "Borna disease virus nucleoprotein (p40) is a major target for CD8(+)-T-cell-mediated immune response". Journal of Virology. 73 (2): 1715–8. doi:10.1128/JVI.73.2.1715-1718.1999. PMC 104005. PMID 9882386.
- ^ Zhang G, Kobayashi T, Kamitani W, Komoto S, Yamashita M, Baba S, Yanai H, Ikuta K, Tomonaga K (November 2003). "Borna disease virus phosphoprotein represses p53-mediated transcriptional activity by interference with HMGB1". Journal of Virology. 77 (22): 12243–51. doi:10.1128/jvi.77.22.12243-12251.2003. PMC 254253. PMID 14581561.
- ^ Kishi M, Arimura Y, Ikuta K, Shoya Y, Lai PK, Kakinuma M (January 1996). "Sequence variability of Borna disease virus open reading frame II found in human peripheral blood mononuclear cells". Journal of Virology. 70 (1): 635–40. doi:10.1128/JVI.70.1.635-640.1996. PMC 189858. PMID 8523585.
- ^ a b c Iwata Y, Takahashi K, Peng X, Fukuda K, Ohno K, Ogawa T, Gonda K, Mori N, Niwa S, Shigeta S (December 1998). "Detection and sequence analysis of borna disease virus p24 RNA from peripheral blood mononuclear cells of patients with mood disorders or schizophrenia and of blood donors". Journal of Virology. 72 (12): 10044–9. doi:10.1128/JVI.72.12.10044-10049.1998. PMC 110530. PMID 9811743.
- ^ Nunes SO, Itano EN, Amarante MK, Reiche EM, Miranda HC, de Oliveira CE, Matsuo T, Vargas HO, Watanabe MA (2008). "RNA from Borna disease virus in patients with schizophrenia, schizoaffective patients, and in their biological relatives". Journal of Clinical Laboratory Analysis. 22 (4): 314–20. doi:10.1002/jcla.20261. PMC 6649126. PMID 18623121.
- ^ Miranda HC, Nunes SO, Calvo ES, Suzart S, Itano EN, Watanabe MA (January 2006). "Detection of Borna disease virus p24 RNA in peripheral blood cells from Brazilian mood and psychotic disorder patients". Journal of Affective Disorders. 90 (1): 43–7. doi:10.1016/j.jad.2005.10.008. PMID 16324750.
- ^ Schwemmle, M. and Lipkin, W.I. (2004) Models and mechanisms of Bornavirus pathogenesis. Drug Discovery Today: Disease Mechanisms 1(2):211–216
- ^ Tomonaga K, Kobayashi T, Ikuta K (April 2002). "Molecular and cellular biology of Borna disease virus infection". Microbes and Infection / Institut Pasteur. 4 (4): 491–500. doi:10.1016/S1286-4579(02)01564-2. PMID 11932200.
- ^ Horie M, Honda T, Suzuki Y, Kobayashi Y, Daito T, Oshida T, Ikuta K, Jern P, Gojobori T, Coffin JM, Tomonaga K (January 2010). "Endogenous non-retroviral RNA virus elements in mammalian genomes". Nature. 463 (7277): 84–7. doi:10.1038/nature08695. PMC 2818285. PMID 20054395.
- ^ He, Mei; An, Tie-Zhu; Teng, Chun-Bo (2014). "Evolution of mammalian and avian bornaviruses". Molecular Phylogenetics and Evolution. 79: 385–91. doi:10.1016/j.ympev.2014.07.006. PMID 25046276.
- ^ "Evolutionary Surprise: Eight Percent of Human Genetic Material Comes from a Virus". ScienceDaily. 2010-01-08.