Normal pressure hydrocephalus
| Normal-pressure hydrocephalus | |
|---|---|
| Other names: Malresorptive hydrocephalus, adult hydrocephalus syndrome[1] | |
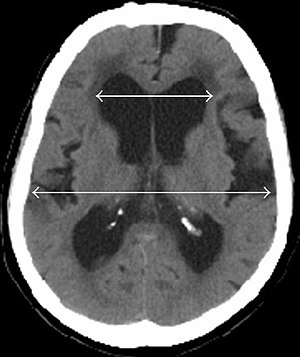 | |
| Evan's index is the ratio of maximum width of the frontal horns to the maximum width of the inner table of the cranium. An Evan's index more than 0.31 indicates hydrocephalus.[2] | |
| Specialty | Neurology |
| Symptoms | Urinary incontinence, dementia, trouble walking[1] |
| Usual onset | Elderly[3] |
| Types | Primary, secondary[1] |
| Causes | Unknown, subarachnoid hemorrhage, head trauma, prior infection, tumor, complications of surgery[3] |
| Diagnostic method | Based on symptom, supported by medical imaging and improvement after lumbar puncture[1] |
| Differential diagnosis | Parkinson disease, Alzheimer disease[3] |
| Treatment | Surgery[3] |
| Prognosis | 80% improve post surgery[1] |
| Frequency | ~1.5% (> 65 years old)[1] |
Normal-pressure hydrocephalus (NPH) is build up of cerebrospinal fluid (CSF) in the ventricles, with near normal cerebrospinal fluid pressure.[4] Symptoms classically include urinary incontinence, dementia, and trouble walking.[1] The trouble walking often involves shuffling or a wide-based gait.[1] Urinary problems may begin as frequent urination.[1] Without treatment, symptoms worsen over time.[3]
While the cause is frequently unknown, it may also occur as a result of a subarachnoid hemorrhage, head trauma, prior infection, tumor, or complications of surgery.[3] The underlying mechanism is believed to involves insufficient absorption of CSF by the arachnoid granulations.[4] It is a type of communicating hydrocephalus.[1] The diagnosis is suspected based on symptom and may be further support by medical imaging and improvement of symptoms after lumbar puncture to drain CSF.[1] Conditions that may present similarly include Parkinson and Alzheimer disease.[3]
Treatment is by surgery to place a ventriculoperitoneal shunt to drain excess CSF into the abdomen to be absorbed by the lining.[3] In those who are unable to have surgery, repeated drainage by lumbar puncture and acetazolamide may be an option.[1] The probability of improvement following surgery is 80%.[1]
NPH most commonly occurs in the elderly.[3] One study found that it affects about 0.2% of those in their 70s and 5.9% of those over 80 years old.[1] Males and females are affected with similar frequency.[1] It is though to represent about 6% of cases of dementia.[1] The disease was first described by Salomón Hakim and Adams in 1965.[5][4]
Signs and symptoms
NPH exhibits a classic findings (known as the Adams triad or Hakim's triad) that consists of gait deviation, dementia, and urinary incontinence (referred to as "wet, wacky, and wobbly" or "weird walking water").
Gait deviations are present in nearly all people and usually the first symptom. This is caused by expansion of the lateral ventricles to impinge on the corticospinal tract motor fibers. The typical gait abnormality in NPH is a broad-based, slow, short-stepped, "stuck to the floor", or "magnetic" movement. The gait abnormalities in NPH may bear resemblance to a gait associated with Parkinson's disease. The gait deviation can be classified as mild, marked, or severe: "marked" is when the patient has difficulty walking because of considerable instability; "severe" is when it is not possible for the patient to walk without aids (such as a cane or a wheeled walker).[6][7]
Dementia presents as progressive cognitive impairment which is present in 60% of people at time of treatment. This is caused by distortions predominantly at the frontal lobe and the subcortex.[8] Initial deficits involve planning, organization, attention, and concentration. Further deficits include difficulty managing finances, taking medications, driving, keeping track of appointments, daytime sleeping, short-term memory impairments, and psychomotor slowing. Late stage features include apathy, reduced drive, slowed thinking, and reduced speech.
Urinary incontinence appears late in the illness, and is present in 50% of people at time of treatment. Urinary dysfunction begins as increased frequency often at night, and progresses to urge incontinence and permanent incontinence.[8]
Pathogenesis
Every day, the body makes roughly 600–700 ml of CSF, and about the same amount is reabsorbed into the bloodstream. Hydrocephalus is due to an imbalance between the amount of fluid produced and its absorption rate. Enlarged ventricles put increased pressure on the adjacent cortical tissue and cause myriad effects in the patient, including distortion of the fibers in the corona radiata. This leads to an increase in intracranial pressure (ICP). The ICP gradually falls, but still remains slightly elevated, and the CSF pressure reaches a high normal level of 150 to 200 mm H2O. Measurements of ICP, therefore, are not usually elevated. Because of this, patients do not exhibit the classic signs that accompany increased intracranial pressure such as headache, nausea, vomiting, or altered consciousness, although some studies have shown pressure elevations to occur intermittently.
The exact pathogenesis is unknown, but consensus on some mechanisms include:
- An imbalance exists between production and resorption of CSF.
- The resistance to CSF outflow is often elevated.
- The disease is not caused by overproduction of CSF or obstruction of CSF flow at the ventricles.
The syndrome is often divided into two groups, primary (also called idiopathic) and secondary, based on cause. The underlying etiology of primary NPH has not yet been identified. Primary NPH affects adults age 40 years or older, most commonly affecting the elderly. Secondary NPH can affect persons of any age and occurs due to conditions such as subarachnoid hemorrhage, meningitis, brain surgery, brain radiation, or traumatic brain injury.
Diagnosis
People with suspected NPH should have typical symptoms in addition to ventricular enlargement on neuroimaging. The international evidenced-based diagnostic criteria for primary NPH are:
- Gradual onset after age 40 years, symptoms duration of ≥ 3–6 months, clinical evidence of gait or balance impairment, and impairment of cognition or urinary incontinence
- Imaging from magnetic resonance imaging (MRI) or computed tomography (CT) is needed to demonstrate enlarged ventricles and no macroscopic obstruction to cerebrospinal fluid flow. Imaging should show an enlargement to at least one of the temporal horns of lateral ventricles, and impingement against the falx cerebri resulting in a callosal angle ≤ 90° on the coronal view, showing evidence of altered brain water content, or normal active flow (which is referred to as "flow void") at the cerebral aqueduct and fourth ventricle.
|
| ||
| Normal pressure hydrocephalus | Brain atrophy | |
|---|---|---|
| Preferable projection | Coronal plane at the level of the posterior commissure of the brain. | |
| Modality in this example | CT | MRI |
| CSF spaces over the convexity near the vertex (red ellipse |
Narrowed convexity ("tight convexity") as well as medial cisterns | Widened vertex (red arrow) and medial cisterns (green arrow) |
| Callosal angle (blue V) | Acute angle | Obtuse angle |
| Most likely cause of leucoaraiosis (periventricular signal alterations, blue arrows |
Transependymal cerebrospinal fluid diapedesis | Vascular encephalopathy, in this case suggested by unilateral occurrence |
MRI scans are preferred. The distinction between normal and enlarged ventricular size by cerebral atrophy is difficult to ascertain. Up to 80% of cases are unrecognized and untreated due to difficulty of diagnosis.[10] Imaging should also reveal the absence of any cerebral mass lesions or any signs of obstructions. Although all people with NPH have enlarged ventricles, not all elderly with enlarged ventricles have primary NPH. Cerebral atrophy can cause enlarged ventricles, as well, and is referred to as hydrocephalus ex vacuo.
The Miller Fisher test involves a high-volume lumbar puncture (LP) with removal of 30–50 ml of CSF. Gait and cognitive function are typically tested just before and within 2–3 hours after the LP to assess for signs of symptomatic improvement. The CSF infusion test and the lumbar test are similar tests to Miller Fisher test. The tests have a positive predictive value over 90%, but a negative predictive value less than 50%. The LP should show normal or mildly elevated CSF pressure. CSF should have normal cell contents, glucose levels, and protein levels.[11][12][13]
Treatment
Shunt
For suspected cases of NPH, CSF shunting is the first-line treatment. The most common type used to treat NPH is ventriculoperitoneal (VP) shunts, which drain CSF fluid to the peritoneal cavity. Adjustable valves allow fine-tuning of CSF drainage. NPH symptoms reportedly improve in 70–90% of patients with CSF shunt. Risk-benefit analyses have shown beyond any doubt that surgery for NPH is far better than conservative treatment or the natural course.[10]
Gait symptoms improve in ≥ 85% patients. Cognitive symptoms improve in up to 80% of patients when surgery is performed early in the disease course. Incontinence improves in up to 80% of patients, but only in ≤ 50–60% of patients with shunt implanted late in disease course. The most likely patients to show improvement are those who show only gait deviation, mild or no incontinence, and mild dementia. The risk of adverse events related to shunt placement is 11%, including shunt failure, infections such as ventriculitis, shunt obstruction, over- or under-drainage, and development of a subdural hematoma.[14][15][16]
Medication
No medications are effective for primary NPH. Acetazolamide and other diuretics are not recommended except for limited use in those who are not candidates for placement of a shunt.
Epidemiology
The majority of cases are primary NPH. The incidence of NPH increases with advancing age, and most patients are over the age of 60. Its prevalence is reported to be less than 1% in persons under the age of 65, and up to 3% for persons aged 65 or older. No difference in incidence is seen between men and women.[8][17][18] Among individuals with dementia, the incidence of NPH is thought to be between 2 and 6%.
See also
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 M Das, J; Biagioni, MC (January 2021). "Normal Pressure Hydrocephalus". PMID 31194404.
{{cite journal}}: Cite journal requires|journal=(help) - ↑ Ishii, Mitsuaki; Kawamata, Toshio; Akiguchi, Ichiro; Yagi, Hideo; Watanabe, Yuko; Watanabe, Toshiyuki; Mashimo, Hideaki (2010). "Parkinsonian Symptomatology May Correlate with CT Findings before and after Shunting in Idiopathic Normal Pressure Hydrocephalus". Parkinson's Disease. 2010: 1–7. doi:10.4061/2010/201089. ISSN 2042-0080. PMC 2951141. PMID 20948890.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 3.7 3.8 "Normal Pressure Hydrocephalus Information Page | National Institute of Neurological Disorders and Stroke". www.ninds.nih.gov. Archived from the original on 1 March 2021. Retrieved 4 March 2021.
- ↑ 4.0 4.1 4.2 Dening, Tom; Thomas, Alan; Taylor, John Paul; Stewart, Robert (2020). Oxford Textbook of Old Age Psychiatry. Oxford University Press. p. 527. ISBN 978-0-19-880729-2. Archived from the original on 2021-08-28. Retrieved 2021-03-04.
- ↑ Adams RD, Fisher CM, Hakim S, Ojemann RG, Sweet WH (July 1965). "Symptomatic Occult Hydrocephalus with Normal Cerebrospinal-Fluid Pressure". The New England Journal of Medicine. 273 (3): 117–26. doi:10.1056/NEJM196507152730301. PMID 14303656.
- ↑ Krauss JK, Faist M, Schubert M, Borremans JJ, Lucking CH, Berger W (2001). "Evaluation of Gait in Normal Pressure Hydrocephalus Before and After Shunting". In Ruzicka E, Hallett M, Jankovic J (eds.). Gait Disorders. Philadelphia, PA: Lippincott Williams & Wilkins. pp. 301–09.
- ↑ Ropper AH, Samuels MA (2009). Adams and Victor's Principles of Neurology (9th ed.). New York: McGraw-Hill Medical.
- ↑ 8.0 8.1 8.2 Younger DS (2005). "Adult Normal Pressure Hydrocephalus". In Younger DS (ed.). Motor Disorders (2nd ed.). Philadelphia, PA: Lippincott Williams & Wilkins. pp. 581–84.
- ↑ Damasceno, Benito Pereira (2015). "Neuroimaging in normal pressure hydrocephalus". Dementia & Neuropsychologia. 9 (4): 350–355. doi:10.1590/1980-57642015DN94000350. ISSN 1980-5764. PMC 5619317. PMID 29213984.
- ↑ 10.0 10.1 Kiefer M, Unterberg A (January 2012). "The differential diagnosis and treatment of normal-pressure hydrocephalus". Deutsches Ärzteblatt International. 109 (1–2): 15–25, quiz 26. doi:10.3238/arztebl.2012.0015. PMC 3265984. PMID 22282714.
- ↑ Tarnaris A, Toma AK, Kitchen ND, Watkins LD (December 2009). "Ongoing search for diagnostic biomarkers in idiopathic normal pressure hydrocephalus". Biomarkers in Medicine. 3 (6): 787–805. doi:10.2217/bmm.09.37. PMID 20477715.
- ↑ Marmarou A, Bergsneider M, Klinge P, Relkin N, Black PM (September 2005). "The value of supplemental prognostic tests for the preoperative assessment of idiopathic normal-pressure hydrocephalus". Neurosurgery. 57 (3 Suppl): S17–28, discussion ii–v. doi:10.1227/01.neu.0000168184.01002.60. PMID 16160426.
- ↑ "NINDS Normal Pressure Hydrocephalus Information Page". National Institute of Neurological Disorders and Stroke. 29 April 2011. Archived from the original on 11 December 2016. Retrieved 13 May 2011.
- ↑ Marmarou A, Young HF, Aygok GA (April 2007). "Estimated incidence of normal pressure hydrocephalus and shunt outcome in patients residing in assisted-living and extended-care facilities". Neurosurgical Focus. 22 (4): E1. doi:10.3171/foc.2007.22.4.2. PMID 17613187.
- ↑ Vanneste J, Augustijn P, Dirven C, Tan WF, Goedhart ZD (January 1992). "Shunting normal-pressure hydrocephalus: do the benefits outweigh the risks? A multicenter study and literature review". Neurology. 42 (1): 54–59. doi:10.1212/wnl.42.1.54. PMID 1734324.
- ↑ Poca MA, Mataró M, Del Mar Matarín M, Arikan F, Junqué C, Sahuquillo J (May 2004). "Is the placement of shunts in patients with idiopathic normal-pressure hydrocephalus worth the risk? Results of a study based on continuous monitoring of intracranial pressure". Journal of Neurosurgery. 100 (5): 855–66. doi:10.3171/jns.2004.100.5.0855. PMID 15137605.
- ↑ Brean A, Eide PK (July 2008). "Prevalence of probable idiopathic normal pressure hydrocephalus in a Norwegian population". Acta Neurologica Scandinavica. 118 (1): 48–53. doi:10.1111/j.1600-0404.2007.00982.x. hdl:10852/27953. PMID 18205881.
- ↑ Tanaka N, Yamaguchi S, Ishikawa H, Ishii H, Meguro K (1 January 2009). "Prevalence of possible idiopathic normal-pressure hydrocephalus in Japan: the Osaki-Tajiri project". Neuroepidemiology. 32 (3): 171–75. doi:10.1159/000186501. PMID 19096225.
External links
- Normal Pressure Hydrocephalus Information Page Archived 2021-03-01 at the Wayback Machine at NINDS
- Normal Pressure Hydrocephalus Archived 2021-08-29 at the Wayback Machine at Cleveland Clinic
| Classification | |
|---|---|
| External resources |
