Setmelanotide
 | |
| Names | |
|---|---|
| Trade names | Imcivree |
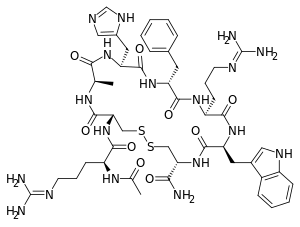
| Other names | RM-493; BIM-22493; IRC-022493; N2-Acetyl-L-arginyl-L-cysteinyl-D-alanyl-L-histidyl-D-phenylalanyl-L-arginyl-L-tryptophyl-L-cysteinamide, cyclic (2-8)-disulfide |
| |
| Clinical data | |
| Main uses | Specific genetic causes of obesity[1][2] |
| Side effects | Injection site reactions, increased skin pigmentation, headache, nausea, diarrhea, abdominal pain, depression[1] |
| Routes of use | Subcutaneous |
| Typical dose | 3 mg OD[1] |
| External links | |
| AHFS/Drugs.com | Monograph |
| Legal | |
| License data |
|
| Legal status | |
| Chemical and physical data | |
| Formula | C49H68N18O9S2 |
| Molar mass | 1117.32 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Setmelanotide, sold under the brand name Imcivree, is a medication used to treat specific genetic causes of obesity.[1][2] This includes significant pro-opiomelanocortin (POMC) deficiency, proprotein convertase subtilisin/kexin type 1 (PCSK1) deficiency, leptin receptor (LEPR) deficiency, and Bardet-Biedl syndrome (BBS).[1] It is used in those at least 6 years old.[2] It is given by injection under the skin.[1]
Common side effects include injection site reactions, increased skin pigmentation, headache, nausea, diarrhea, abdominal pain, and depression.[1] Other side effects may include sexual arousal and thoughts of suicide.[1] Use in not recommended in pregnancy or breast feeding.[1] It is a melanocortin 4 (MC4) receptor agonist.[1]
Setmelanotide was approved for medical use in the United States in 2020 and Europe in 2021.[1][2] In the United States it costs about 33,000 USD per month as of 2022.[4]
Medical uses
Setmelanotide is indicated for chronic weight management (weight loss and weight maintenance for at least one year) in people six years and older with obesity due to three rare genetic conditions: pro-opiomelanocortin (POMC) deficiency, proprotein subtilisin/kexin type 1 (PCSK1) deficiency, and leptin receptor (LEPR) deficiency confirmed by genetic testing demonstrating variants in POMC, PCSK1, or LEPR genes considered pathogenic (causing disease), likely pathogenic, or of uncertain significance.[1][3][2]
In three small studies 40% to 80% of people had a 10% decrease in their weight.[2]
Setmelanotide is not approved for obesity due to suspected POMC, PCSK1, or LEPR deficiency with variants classified as benign (not causing disease) or likely benign or other types of obesity, including obesity associated with other genetic syndromes and general (polygenic) obesity.[3]
Dosage
It started at a dose of 1 mg in those 6 to 12 years old and a dose of 2 mg in those 12 and over.[1] This is given once per day for two weeks.[1] This is than increased to 3 mg once per day.[1]
Pharmacology
Setmelanotide binds to and activates MC4 receptors in the paraventricular nucleus (PVN) of the hypothalamus and in the lateral hypothalamic area (LHA), areas involved in the regulation of appetite, and this action is thought to underlie its appetite suppressant effects.[5] In addition to reducing appetite, setmelanotide increases resting energy expenditure in both obese animals and humans.[6] Importantly, unlike certain other MC4 receptor agonists, such as LY-2112688, setmelanotide has not been found to produce increases in heart rate or blood pressure.[7]
Setmelanotide has been reported to possess the following activity profile (cAMP, EC50): MC4 (0.27 nM) > MC3 (5.3 nM) ≈ MC1 (5.8 nM) > MC5 (1600 nM) ≟ MC2 (>1000 nM).[8] (19.6-fold selectivity for MC4 over MC3, the second target of highest activity.)
History
Setmelanotide was invented at Ipsen by Jesse Z. Dong.[9] It was initially known as BIM-22493 and then as RM-493 and IRC-022493.
It was evaluated in two one-year clinical studies.[3] The first study enrolled 10 participants with obesity and confirmed or suspected POMC or PCSK1 deficiency while the second study enrolled 11 participants with obesity and confirmed or suspected LEPR deficiency; all participants were six years or older.[3] Most participants had lost more than 10% of their initial body weight after a year of treatment.[3] Some participants also reported feeling less hungry.[3]
The U.S. Food and Drug Administration (FDA) granted the application for setmelanotide orphan disease designation, breakthrough therapy designation, and priority review.[3] The FDA granted the approval of Imcivree to Rhythm Pharmaceutical, Inc.[3][10] The U.S. Food and Drug Administration (FDA) considers it to be a first-in-class medication.[11][12] Setmelanotide is the first FDA-approved treatment for these genetic conditions.[3]
Society and culture
Legal status
Setmelanotide was approved for medical use in the United States in November 2020.[10][12]
Setmelanotide was approved for medical use in the European Union in July 2021.[2]
Research
Setmelanotide is a peptide drug and investigational anti-obesity medication which acts as a selective agonist of the MC4 receptor;[13][7] the structure of the bound complex has recently been determined.[14] Its peptide sequence is Ac-Arg-Cys(1)-D-Ala-His-D-Phe-Arg-Trp-Cys(1)-NH2. It was first discovered at Ipsen and is being developed by Rhythm Pharmaceuticals for the treatment of obesity and diabetes.[13] In addition, Rhythm Pharmaceuticals is conducting trials of setmelanotide for the treatment of Prader–Willi syndrome (PWS), a genetic disorder which includes MC4 receptor deficiency and associated symptoms such as excessive appetite and obesity.[15] As of December 2014, the drug is in phase II clinical trials for obesity and PWS.[13][16][17][needs update] So far, preliminary data has shown no benefit of Setmelanotide in Prader-Willi syndrome.[18]
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 "Imcivree- setmelanotide solution". DailyMed. Archived from the original on 21 October 2021. Retrieved 25 December 2020.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 "Imcivree EPAR". European Medicines Agency (EMA). 19 May 2021. Archived from the original on 23 July 2021. Retrieved 22 July 2021. Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 3.7 3.8 3.9 "FDA approves first treatment for weight management for people with certain rare genetic conditions". U.S. Food and Drug Administration (FDA) (Press release). 27 November 2020. Archived from the original on 27 November 2020. Retrieved 27 November 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "Imcivree". Archived from the original on 11 November 2022. Retrieved 3 November 2022.
- ↑ Kim GW, Lin JE, Blomain ES, Waldman SA (January 2014). "Antiobesity pharmacotherapy: new drugs and emerging targets". Clinical Pharmacology and Therapeutics. 95 (1): 53–66. doi:10.1038/clpt.2013.204. PMC 4054704. PMID 24105257.
- ↑ Chen KY, Muniyappa R, Abel BS, Mullins KP, Staker P, Brychta RJ, et al. (April 2015). "RM-493, a melanocortin-4 receptor (MC4R) agonist, increases resting energy expenditure in obese individuals". The Journal of Clinical Endocrinology and Metabolism. 100 (4): 1639–45. doi:10.1210/jc.2014-4024. PMC 4399297. PMID 25675384.
- ↑ 7.0 7.1 Kievit P, Halem H, Marks DL, Dong JZ, Glavas MM, Sinnayah P, et al. (February 2013). "Chronic treatment with a melanocortin-4 receptor agonist causes weight loss, reduces insulin resistance, and improves cardiovascular function in diet-induced obese rhesus macaques". Diabetes. 62 (2): 490–7. doi:10.2337/db12-0598. PMC 3554387. PMID 23048186.
- ↑ Muniyappa R, Chen K, Brychta R, Abel B, Mullins K, Staker P, et al. (June 2014). "A Randomized, Double-Blind, Placebo-Controlled, Crossover Study to Evaluate the Effect of a Melanocortin Receptor 4 (MC4R) Agonist, RM-493, on Resting Energy Expenditure (REE) in Obese Subjects" (PDF). Endocrine Reviews. Rhythm Pharmaceuticals. 35 (3). Archived (PDF) from the original on 2015-05-22. Retrieved 2015-05-21.
- ↑ US Patent US 8,039,435 B2, "Melanocortin Receptor Ligands", October 18, 2011, Inventors: Zheng Xin Dong, Jacques-Pierre Moreau
- ↑ 10.0 10.1 "Drug Approval Package: Imcivree". U.S. Food and Drug Administration (FDA). 23 December 2020. Archived from the original on 23 January 2021. Retrieved 18 January 2021.
- ↑ "New Drug Therapy Approvals 2020". U.S. Food and Drug Administration (FDA). 31 December 2020. Archived from the original on 18 January 2021. Retrieved 17 January 2021.
- ↑ 12.0 12.1 "FDA approves first treatment for weight management". U.S. Food and Drug Administration (FDA). 30 September 2021. Archived from the original on 13 February 2022. Retrieved 12 February 2022.
- ↑ 13.0 13.1 13.2 Lee EC, Carpino PA (2015). "Melanocortin-4 receptor modulators for the treatment of obesity: a patent analysis (2008-2014)". Pharmaceutical Patent Analyst. 4 (2): 95–107. doi:10.4155/ppa.15.1. PMID 25853469.
- ↑ Israeli, Hadar; Degtjarik, Oksana; Fierro, Fabrizio; Chunilal, Vidicha; Gill, Amandeep Kaur; Roth, Nicolas J.; Botta, Joaquin; Prabahar, Vadivel; Peleg, Yoav; Chan, Li F.; Ben-Zvi, Danny (2021-05-21). "Structure reveals the activation mechanism of the MC4 receptor to initiate satiation signaling". Science. 372 (6544): 808–814. Bibcode:2021Sci...372..808I. doi:10.1126/science.abf7958. ISSN 0036-8075. PMID 33858992. S2CID 233260097. Archived from the original on 2022-03-25. Retrieved 2022-06-30.
- ↑ "Obesity and Diabetes Caused by Genetic Deficiencies in the MC4 Pathway". Rhythm Pharmaceuticals. Archived from the original on 2015-06-14. Retrieved 2015-05-21.
- ↑ Jackson VM, Price DA, Carpino PA (August 2014). "Investigational drugs in Phase II clinical trials for the treatment of obesity: implications for future development of novel therapies". Expert Opinion on Investigational Drugs. 23 (8): 1055–66. doi:10.1517/13543784.2014.918952. PMID 25000213. S2CID 23198484.
- ↑ "RM-493: A First-in-Class, Phase 2-Ready MC4 Agonist: A New Drug Class for the Treatment of Obesity and Diabetes". Rhythm Pharmaceuticals. Archived from the original on 2015-06-14. Retrieved 2015-05-21.
- ↑ Duis J, van Wattum PJ, Scheimann A, Salehi P, Brokamp E, Fairbrother L, et al. (March 2019). "A multidisciplinary approach to the clinical management of Prader-Willi syndrome". Molecular Genetics & Genomic Medicine. 7 (3): e514. doi:10.1002/mgg3.514. PMC 6418440. PMID 30697974.
External links
| External sites: | |
|---|---|
| Identifiers: |
|
- Clinical trial number NCT02896192 for "Setmelanotide for the Treatment of Early-Onset POMC Deficiency Obesity" at ClinicalTrials.gov
- Clinical trial number NCT03287960 for "Setmelanotide for the Treatment of LEPR Deficiency Obesity" at ClinicalTrials.gov
- Pages using duplicate arguments in template calls
- Wikipedia articles incorporating the PD-notice template
- Drugs with non-standard legal status
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without UNII source
- Drugs missing an ATC code
- Drug has EMA link
- Articles containing unverified chemical infoboxes
- Wikipedia articles in need of updating from November 2017
- Articles with invalid date parameter in template
- All Wikipedia articles in need of updating
- Anorectics
- Anti-diabetic drugs
- Antiobesity drugs
- Breakthrough therapy
- Melanocortin receptor agonists
- Orphan drugs
- Peptides
- RTT