Retapamulin
 | |
 | |
| Names | |
|---|---|
| Trade names | Altabax, Altargo |
| |
| Clinical data | |
| Pregnancy category |
|
| Routes of use | Topical (ointment) |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a607049 |
| Legal | |
| License data |
|
| Legal status | |
| Pharmacokinetics | |
| Bioavailability | Low |
| Protein binding | 94% |
| Metabolism | Liver, CYP3A4-mediated |
| Elimination half-life | Undetermined |
| Excretion | Undetermined |
| Chemical and physical data | |
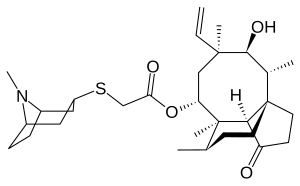
| Formula | C30H47NO4S |
| Molar mass | 517.77 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Retapamulin, sold as the brand name Altabax among others, is a antibiotic which is applied to the skin to threat impetigo, a bacterial skin infection.[1] It is not useful against MRSA.[1]
Common side effects include irritation at the site it is used.[1] Other side effects may include allergic reactions such as angioedema.[1] It generally works by stopping bacterial growth by inhibiting the bacterial ribosome.[1]
Retapamulin was approved for medical use in the United States and Europe in 2007.[1][2] It; however, was withdrawn from the market in 2019 in Europe.[2] In the United States 15 grams of 1% cream costs about 325 USD as of 2021.[3] It was original made from a fungus.[4]
Medical uses
Retapamulin is used for the topical treatment of impetigo due to Staphylococcus aureus (methicillin-susceptible only) or Streptococcus pyogenes.[5]
Dosage
It is used twice per day for 5 days.[4]
Contraindications
None yet reported.[5]
Side effects
The most common reported adverse reaction was irritation at the application site.[5]
Pharmacology
Mechanism of action
Retapamulin is an antibacterial agent, specifically a protein synthesis inhibitor. The medication selectively inhibits bacterial protein synthesis by interacting at a site on the 50S subunit of the bacterial ribosome through an interaction that differs from other antibiotics.[5]
Pharmacokinetics
Systemic exposure following topical application through intact skin is low.[5]
History
It is the first drug in the new class of pleuromutilin antibiotics to be approved for human use.
It was withdrawn from the market in 2019 by Glaxo Group as they did not consider it commercially viable.[6]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 "Retapamulin Monograph for Professionals". Drugs.com. Archived from the original on 6 August 2020. Retrieved 21 July 2021.
- ↑ 2.0 2.1 "Altargo". Archived from the original on 9 April 2021. Retrieved 21 July 2021.
- ↑ "Retapamulin Prices, Coupons & Savings Tips - GoodRx". GoodRx. Retrieved 21 July 2021.
- ↑ 4.0 4.1 Hartman-Adams, Holly; Banvard, Christine; Juckett, Gregory (15 August 2014). "Impetigo: Diagnosis and Treatment". American Family Physician. 90 (4): 229–235. ISSN 0002-838X. Archived from the original on 24 May 2021. Retrieved 21 July 2021.
- ↑ 5.0 5.1 5.2 5.3 5.4 Borrza, S.; Philippi, E., eds. (2007). Physicians' Desk Reference (62nd ed.). pp. 1318–20. ISBN 978-1-56363-660-8.
- ↑ "Altargo Withdrawal of the marketing authorisation in the European Union" (PDF). Archived (PDF) from the original on 18 March 2019. Retrieved 21 July 2021.
External links
| Identifiers: |
|
|---|
- Pages using duplicate arguments in template calls
- Articles with changed InChI identifier
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without UNII source
- Drugs missing an ATC code
- Drug has EMA link
- Drugboxes which contain changes to verified fields
- Drugboxes which contain changes to watched fields
- Articles with changed ChemSpider identifier
- Articles with changed EBI identifier
- Pleuromutilin antibiotics
- GlaxoSmithKline brands
- Secondary alcohols
- Ketones
- Carboxylate esters
- Thioethers
- Nitrogen heterocycles
- RTT