Lefamulin
 | |
| Names | |
|---|---|
| Trade names | Xenleta |
| Other names | Lefamulin acetate, BC-3781 |
| |
| Clinical data | |
| Drug class | Antibiotic (pleuromutilin)[1] |
| Main uses | Pneumonia[1] |
| Side effects | Diarrhea, nausea, pain at the site of injection, liver inflammation[2] |
| WHO AWaRe | |
| Routes of use | Intravenous, by mouth |
| External links | |
| AHFS/Drugs.com | Monograph |
| Legal | |
| License data | |
| Legal status | |
| Chemical and physical data | |
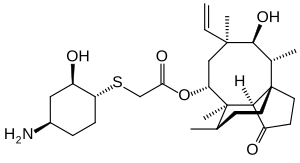
| Formula | C28H45NO5S |
| Molar mass | 507.73 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Lefamulin, sold under the brand name Xenleta, is an antibiotic used to treat community-acquired pneumonia.[1] It is used when other antibiotics are not appropriate.[1] It is effective against a number of bacteria including MRSA.[2] It is taken by mouth or by injection into a vein.[1]
Common side effects include diarrhea, nausea, pain at the site of injection, and liver inflammation.[2] Other side effects may include QT prolongation and Clostridioides difficile infection.[2] Use during pregnancy may harm the baby.[2] It is a pleuromutilin antibiotic and works by blocking the production of proteins from bacterial RNA.[1]
Lefamulin was approved for medical use in the United States in 2019 and Europe in 2020.[3][1] In the United States a 5 day course of treatment costs about 1450 USD as of 2021.[4] While it is approved in Europe, it is not commercially available there as of 2021.[5]
Medical uses
Lefamulin is used to treat adults with community-acquired bacterial pneumonia.[6][7][1] It was also investigated for treatment of acute bacterial skin and skin-structure infections (ABSSSI).[8]
Spectrum of activity
Lefamulin has in vitro activity against Streptococcus viridans, Moraxella catarrhalis, Enterococcus faecium, methicillin-resistant Staphylococcus aureus (MRSA), among other bacteria.[9][10]
Dosage
It is taken by mouth at a dose of 600 mg twice per day for 5 days.[1] Or by injection at a dose of 150 mg twice per day.[1]
History
It was developed by Nabriva Therapeutics and approved in the United States in 2019.[6] It was granted fast track status by the US Food and Drug Administration (FDA) in 2014. Although pleuromutilin antibiotics were first developed in the 1950s, lefamulin is the first to be used for systemic treatment of bacterial infections in humans.[11]
Society and culture
Legal status
Lefamulin was approved for medical use in the United States in August 2019, and in the European Union in July 2020.[6][1][12] Chinese manufacturer APICDMO recently announced that it has mass-produced Lefamulin API, with a monthly production of 500kg.[13]
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 "Xenleta EPAR". European Medicines Agency. 26 May 2020. Archived from the original on 9 January 2021. Retrieved 24 September 2020.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 "Xenleta- lefamulin acetate injection, solution citric buffered normal saline- anhydrous citric acid injection, solution Xenleta- lefamulin acetate tablet, coated". DailyMed. 12 February 2020. Archived from the original on 6 August 2020. Retrieved 24 September 2020.
- ↑ "Lefamulin Monograph for Professionals". Drugs.com. Archived from the original on 24 January 2021. Retrieved 21 November 2021.
- ↑ "Xenleta Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 19 April 2021. Retrieved 21 November 2021.
- ↑ "Lefamulin". SPS - Specialist Pharmacy Service. 14 January 2016. Archived from the original on 21 November 2021. Retrieved 21 November 2021.
- ↑ 6.0 6.1 6.2 "FDA approves new antibiotic to treat community-acquired bacterial pneumonia". U.S. Food and Drug Administration (FDA) (Press release). 19 August 2019. Archived from the original on 20 November 2019. Retrieved 19 November 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "Drug Trials Snapshots: Xenleta". U.S.Food and Drug Administration (FDA). 4 September 2019. Archived from the original on 20 November 2019. Retrieved 19 November 2019.
- ↑ Zeitlinger, M; Schwameis, R; Burian, A; Burian, B; Matzneller, P; Müller, M; Wicha, W. W.; Strickmann, D. B.; Prince, W (2016). "Simultaneous assessment of the pharmacokinetics of a pleuromutilin, lefamulin, in plasma, soft tissues and pulmonary epithelial lining fluid". Journal of Antimicrobial Chemotherapy. 71 (4): 1022–6. doi:10.1093/jac/dkv442. PMID 26747098.
- ↑ H. Spreitzer (23 May 2016). "Neue Wirkstoffe - Lefamulin". Österreichische Apothekerzeitung (in Deutsch) (11/2016).
- ↑ Mendes, R. E.; Farrell, D. J.; Flamm, R. K.; Talbot, G. H.; Ivezic-Schoenfeld, Z; Paukner, S; Sader, H. S. (2016). "In vitro activity of lefamulin tested against Streptococcus pneumoniae with defined serotypes, including multidrug-resistant isolates causing lower respiratory tract infections in the United States". Antimicrobial Agents and Chemotherapy. 60 (7): AAC.00627–16. doi:10.1128/AAC.00627-16. PMC 4914675. PMID 27161634.
- ↑ Veve, MP; Wagner, JL (September 2018). "Lefamulin: Review of a Promising Novel Pleuromutilin Antibiotic". Pharmacotherapy. 38 (9): 935–946. doi:10.1002/phar.2166. PMID 30019769. S2CID 51679073.
- ↑ "Drug Approval Package: Xenleta". U.S.Food and Drug Administration (FDA). 26 September 2019. Archived from the original on 31 October 2020. Retrieved 24 September 2020.
- ↑ "Lefamulin(1061337-51-6)". apicdmo.com. Archived from the original on 26 October 2021. Retrieved 12 October 2021.
External links
| Identifiers: |
|
|---|
- "Lefamulin". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 3 August 2020. Retrieved 12 October 2021.
- Pages using duplicate arguments in template calls
- Wikipedia articles incorporating the PD-notice template
- CS1 Deutsch-language sources (de)
- Use dmy dates from November 2019
- Articles with invalid date parameter in template
- Drugs with non-standard legal status
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without UNII source
- Drugs missing an ATC code
- Articles containing unverified chemical infoboxes
- Pleuromutilin antibiotics
- RTT
- All stub articles
- Antibiotic stubs