Omega-3 acid ethyl esters
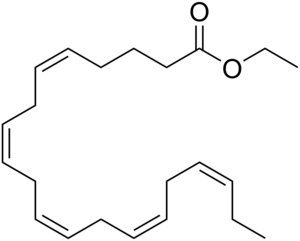 Chemical structure of ethyl eicosapentaenoate, an important omega-3 acid ethyl ester | |
| Combination of | |
|---|---|
| Eicosapentaenoic acid | Antilipemic agent |
| Docosahexaenoic acid | Antilipemic agent |
| Names | |
| Trade names | Lovaza, Omtryg, others |
| Clinical data | |
| Drug class | Omega-3 fatty acid |
| Main uses | High blood triglycerides[1] |
| Side effects | Burping, nausea, upset abdomen[1][2] |
| Pregnancy category | |
| Routes of use | By mouth |
| Defined daily dose | not established[4] |
| External links | |
| AHFS/Drugs.com | Monograph |
| Legal | |
| License data |
|
| Legal status | |
Omega-3 acid ethyl esters are the omega-3 fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) found in fish oil.[1] Together with dietary changes, they are used to treat high blood triglycerides which may reduce the risk of pancreatitis.[1][6] They are generally less preferred than statins and use is not recommended by NHS Scotland as the evidence does not support a decreased risk of heart disease.[1][2][7] Omega-3 acid ethyl esters are taken by mouth.[1]
Common side effects include burping, nausea, and an upset abdomen.[1][2] Serious side effects may include liver problems and anaphylaxis.[1] While use in pregnancy has not been well studied, some omega-3 fatty acids appear beneficial.[3] How it works is not entirely clear.[1]
Omega-3 acid ethyl ester medicines were approved for medical use in the European Union in 2000, and in the United States in 2004.[7][8][1] It is available as a generic medication and over the counter.[2][1] A one-month supply in the United Kingdom costs the NHS about £6 as of 2019.[2] In the United States the wholesale cost of this amount is about 7.50 USD.[9] In 2017, it was the 158th most commonly prescribed medication in the United States, with more than three million prescriptions.[10][11]
Medical use
Omega-3 acid ethyl esters are used in addition to changes in diet to reduce triglyceride levels in adults with severe (≥ 500 mg/dL) hypertriglyceridemia.[12] In the European Union and other major markets outside the US, omega-3 acid ethyl esters are indicated for hypertriglyceridemia by itself, or in combination with a statin for people with mixed dyslipidemia.[5][7]
Intake of large doses (2.0 to 4.0 g/day) of long-chain omega-3 fatty acids as prescription drugs or dietary supplements are generally required to achieve significant (> 15%) lowering of triglycerides, and at those doses the effects can be significant (from 20% to 35% and even up to 45% in individuals with levels greater that 500 mg/dL). It appears that both eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) lower triglycerides, but DHA appears to raise LDL-C ("bad cholesterol") more than EPA, while DHA raises HDL-C ("good cholesterol") while EPA does not.[13]
Dosage
The defined daily dose is not established[4]
Other fish-oil based drugs
There are other omega-3 fish oil based prescription drugs on the market that have similar uses and mechanisms of action.[14]
- Ethyl eicosapentaenoic acid (Vascepa). EPA-only ethyl esters.[15]
- Omega-3 carboxylic acids (Epanova). This product contains free fatty acids, not ethyl esters.[16]
Dietary supplements
There are many fish oil dietary supplements on the market.[17] There appears to be little difference in effect between dietary supplement and prescription forms of omega-3 fatty acids as to ability to lower triglycerides, but the ethyl ester products work less well when taken on an empty stomach or with a low-fat meal.[13] The ingredients of dietary supplements are not as carefully controlled as prescription products and have not been tested in clinical trials as such drugs have.[18] Prescription omega-3 products are more concentrated, requiring fewer softgels for the same daily dose.[17]
Side effects
Special caution should be taken with people who have fish and shellfish allergies.[12] In addition, as with other omega-3 fatty acids, taking omega-3 acid ethyl esters puts people who are on anticoagulants at risk for prolonged bleeding time.[12][13]
Side effects include stomach ache, burping, and a bad taste; some people on very high doses (8g/day) in clinical trials had atrial fibrillation.[12]
Omega-3 acid ethyl esters have not been tested in pregnant women and are rated pregnancy category C; it is excreted in breast milk and the effects on infants are not known.[12]
Pharmacology
After ingestion, omega-3 acid ethyl esters are metabolized mostly in the liver like other dietary fatty acids.[5]
Mechanism of action
Omega-3 acid ethyl esters, like other omega-3 fatty acid based drugs, appears to reduce production of triglycerides in the liver, and to enhance clearance of triglycerides from circulating very low-density lipoprotein (VLDL) particles; the way it does that is not clear, but potential mechanisms include increased breakdown of fatty acids; inhibition of diglyceride acyltransferase which is involved in biosynthesis of triglycerides in the liver; and increased activity of lipoprotein lipase in blood.[14] [5] The synthesis of triglycerides is reduced in the liver as EPA and DHA are poor substrates for the enzymes responsible for triglyceride synthesis.
Physical and chemical properties
The active ingredient is concentrated omega-3 acid ethyl esters that are made from fish body oils that are purified and esterified.[19] For the Lovaza product, each 1000 mg softgel capsule contains 840 mg omega-3 fatty acids: eicosapentaenoic acid ethyl ester (460 mg) and docosahexaenoic acid ethyl ester (380 mg).[5]
History
Pronova BioPharma ASA had its roots in Norway's codfish liver oil industry; it was founded in 1991 as a spinout from the JC Martens company, which in turn was founded in 1838 in Bergen, Norway.[20] Pronova developed the concentrated omega-3 acid ethyl esters formulation that is the active pharmaceutical ingredient of Lovaza.[19]
It won approvals to market the drug, called Omacor in Europe (and initially in the US) in several European countries in 2001 after conducting a three and a half year trial in 11,000 subjects;[21] it partnered with other companies like Pierre Fabre in France.[22] In 2004 Pronova licensed the US and Puerto Rican rights to Reliant Therapeutics, the business model of which was in-licensing cardiovascular drugs.[23] In that same year, Reliant and Pronova won FDA approval for the drug[24] and it was launched in the US and Europe in 2005; global sales in 2005 were $144M and by 2008 they were $778M.[25] In 2007 GlaxoSmithKline acquired Reliant for $1.65 billion in cash.[26]
In 2009 generic companies Teva Pharmaceuticals and Par Pharmaceutical made clear their intentions to file Abbreviated New Drug Applications to bring generics to market, and in April 2009 Pronova sued them from infringing the key US patents covering Lovaza, US 5,656,667 (due to expire in April 2017) US 5,502,077 (exp March 2013) and in May 2012 a district court ruled in Pronova's favor, saying that the patents were valid.[27][28][29] The generic companies appealed and in September 2013 the Federal Circuit reversed, saying that because more than one year before Pronova's predecessor company applied for a patent, it had sent samples of the fish oil used in Lovaza to a researcher for testing, and this constituted "public use" that made the patent invalid.[30][31] Generic versions of Lovaza were introduced in America in April 2014.[32]
Pronovo has continued to manufacture the ingredients in Lovaza, and in 2012 BASF announced it would acquire Pronova for $844 million,[33] and the deal closed in 2013.[34]
Brand names
- Lovaza (US)/Omacor Europe), sold by GlaxoSmithKline in the US; created and manufactured by Pronova[35] It was approved in the United States in 2004.[36]
- Omtryg is another brand of omega-3 acid ethyl esters developed by Trygg Pharma, Inc. and approved by the FDA in 2004.[37]
- As of March 2016 there were four additional generic versions.[38]
Society and culture
Cost
A one-month supply in the United Kingdom costs the NHS about £6 as of 2019.[2]In the United States the wholesale cost of this amount is about 7.50 USD.[9] In 2017, it was the 158th most commonly prescribed medication in the United States, with more than three million prescriptions.[10][11]
-
Omega-3 acid ethyl esters costs (US)
-
Omega-3 acid ethyl esters prescriptions (US)
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 "Omega-3-acid Ethyl Esters Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Archived from the original on 13 April 2019. Retrieved 13 April 2019.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 British national formulary : BNF 76 (76 ed.). Pharmaceutical Press. 2018. pp. 206–207. ISBN 9780857113382.
- ↑ 3.0 3.1 3.2 "Omega-3 polyunsaturated fatty acids Use During Pregnancy". Drugs.com. Archived from the original on 13 April 2019. Retrieved 13 April 2019.
- ↑ 4.0 4.1 "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 1 July 2021. Retrieved 9 September 2020.
- ↑ 5.0 5.1 5.2 5.3 5.4 "Summary of Product Characteristics (SmPC) - (emc)". Omacor. 11 December 2019. Archived from the original on 13 August 2019. Retrieved 15 May 2020.
- ↑ Karalis DG (February 2017). "A Review of Clinical Practice Guidelines for the Management of Hypertriglyceridemia: A Focus on High Dose Omega-3 Fatty Acids". Advances in Therapy. 34 (2): 300–323. doi:10.1007/s12325-016-0462-y. PMC 5331085. PMID 27981496.
- ↑ 7.0 7.1 7.2 "Omega-3 fatty acid medicines". European Medicines Agency. 17 September 2018. Archived from the original on 13 April 2019. Retrieved 13 April 2019.
- ↑ "Drug Approval Package: Omacor (Omega-3-Acid Ethyl Esters) NDA #021654". U.S. Food and Drug Administration (FDA). 24 December 1999. Archived from the original on 2 February 2020. Retrieved 15 May 2020.
- ↑ 9.0 9.1 "NADAC as of 2019-02-27". Centers for Medicare and Medicaid Services. Archived from the original on 2019-03-06. Retrieved 3 March 2019.
- ↑ 10.0 10.1 "The Top 300 of 2020". ClinCalc. Archived from the original on 12 February 2021. Retrieved 11 April 2020.
- ↑ 11.0 11.1 "Omega-3-acid Ethyl Esters - Drug Usage Statistics". ClinCalc. Archived from the original on 8 July 2020. Retrieved 11 April 2020.
- ↑ 12.0 12.1 12.2 12.3 12.4 Lovaza Label Archived 2017-01-18 at the Wayback Machine Revised: March 2016. Updated labels available at FDA website here Archived 2021-08-28 at the Wayback Machine
- ↑ 13.0 13.1 13.2 Jacobson TA, Maki KC, Orringer CE, Jones PH, Kris-Etherton P, Sikand G, et al. (NLA Expert Panel) (2015). "National Lipid Association Recommendations for Patient-Centered Management of Dyslipidemia: Part 2". Journal of Clinical Lipidology. 9 (6 Suppl): S1–122.e1. doi:10.1016/j.jacl.2015.09.002. PMID 26699442.
- ↑ 14.0 14.1 Weintraub HS (November 2014). "Overview of prescription omega-3 fatty acid products for hypertriglyceridemia". Postgraduate Medicine. 126 (7): 7–18. doi:10.3810/pgm.2014.11.2828. PMID 25387209.
- ↑ CenterWatch Vascepa (icosapent ethyl) Archived 2019-08-05 at the Wayback Machine Page accessed March 31, 2016
- ↑ "Epanova (omega-3-carboxylic acids)". CenterWatch. Archived from the original on 25 December 2014. Retrieved 15 December 2014.
- ↑ 17.0 17.1 Ito MK (December 2015). "A Comparative Overview of Prescription Omega-3 Fatty Acid Products". Pharmacy and Therapeutics. 40 (12): 826–57. PMC 4671468. PMID 26681905.
- ↑ Sweeney MET. Hypertriglyceridemia Pharmacologic Therapy Archived 2018-04-08 at the Wayback Machine for Medscape Drugs & Diseases, Ed. Khardori R. Updated: Apr 14, 2015, page accessed April 1, 2016
- ↑ 19.0 19.1 Adis Insight Omega-3 ethylester concentrate Archived 2016-04-14 at the Wayback Machine Page accessed March 31, 2016
- ↑ Epax History Archived 2016-01-23 at the Wayback Machine Page accessed March 31, 2016
- ↑ Pharma Times. March 22, 2001. Pronova wins Omacor approval Archived 2016-04-14 at the Wayback Machine
- ↑ Staff, ICIS Chemical Business. 26 March 2001 Omacor approved Archived 2016-04-13 at the Wayback Machine
- ↑ Amanda Ernst for Law360 November 27, 2007 German Court Invalidates Omega-3 Drug Patent Archived 2016-04-13 at the Wayback Machine
- ↑ VHA Pharmacy Benefits Management Strategic Healthcare Group and the Medical Advisory Panel. October 2005 National PBM Drug Monograph Omega-3-acid ethyl esters (Lovaza, formerly Omacor) Archived 2016-12-22 at the Wayback Machine
- ↑ Staff, Pharmaceutical Technology. Pronova Biopharma Pharmaceutical Production Facility, Denmark Archived 2016-04-07 at the Wayback Machine. Page accessed March 31, 2016
- ↑ Staff, Genetic Engineering News. Nov 21, 2007. GSK to Acquire Reliant Pharmaceuticals for $1.65B Archived 2016-04-20 at the Wayback Machine
- ↑ U.S. Patent 5,656,667
- ↑ U.S. Patent 5,502,077
- ↑ Pronova BioPharma May 29, 2012 Press Release: US District Court rules in Pronova BioPharma's favour on Lovaza(TM) patents Archived 2021-08-28 at the Wayback Machine
- ↑ Ryan Davis for Law360 September 12, 2013 Fed. Circ. Nixes Pronova's Patent Win Against Teva, Par Archived 2016-04-13 at the Wayback Machine
- ↑ United States Court of Appeals for the Federal Circuit. Pronova Biopharma Norge AS vs Teva Pharmaceuticals, Inc and Par Pharmaceutical. 2012-1498, -1499. Decided September 12, 2013 Federal Circuit Decision Archived 2016-04-24 at the Wayback Machine
- ↑ Tracy Staton for FiercePharmaMarketing April 9, 2014 Teva puts GSK and Amarin on notice with generic Lovaza launch Archived 2016-03-31 at the Wayback Machine
- ↑ Ryan McBride for FierceBiotech. November 21, 2012 BASF to snap up fish oil drugmaker Pronova BioPharma in $844M buyout Archived 2013-01-22 at the Wayback Machine
- ↑ Eric Palmer for FierceManufacturing. January 22, 2013 BASF lands Pronova making it a top omega-3 maker Archived 2014-07-23 at the Wayback Machine
- ↑ University of Utah Pharmacy Services (August 15, 2007) "Omega-3-acid Ethyl Esters Brand Name Changed from Omacor to Lovaza" Archived March 3, 2016, at the Wayback Machine
- ↑ Ito, MK (2015). "A Comparative Overview of Prescription Omega-3 Fatty Acid Products". P & T. 40 (12): 826–857. PMC 4671468. PMID 26681905.
- ↑ Omtryg Label Archived 2016-09-29 at the Wayback Machine Revised April 2014
- ↑ FDA Omega-3 acid ethyl esters products Archived 2015-09-05 at the Wayback Machine Page accessed March 31, 2016
External links
| Identifiers: |
|
|---|
- "Lovaza". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 2020-09-21. Retrieved 2020-05-15.
- "Omega-3-acid ethyl esters". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 2020-10-23. Retrieved 2020-05-15.
- Pages using duplicate arguments in template calls
- Webarchive template wayback links
- Drugs with non-standard legal status
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Drugs missing an ATC code
- Drug has EMA link
- Articles containing unverified chemical infoboxes
- Drugs that are a combination of chemicals
- Drugs not assigned an ATC code
- Drugs acting on the cardiovascular system
- Fatty acid esters
- Fish products
- GlaxoSmithKline brands
- Hypolipidemic agents
- RTT

