Chromosome 5q deletion syndrome
| Chromosome 5q deletion syndrome | |
|---|---|
| Other names: Chromosome 5q monosomy, 5q- syndrome | |
 | |
| Photomicrograph of bone marrow showing abnormal mononuclear megakaryocytes typical of 5q- syndrome | |
Chromosome 5q deletion syndrome is an acquired, hematological disorder characterized by loss of part of the long arm (q arm, band 5q33.1) of human chromosome 5 in bone marrow myelocyte cells. This chromosome abnormality is most commonly associated with the myelodysplastic syndrome.
It should not be confused with "partial trisomy 5q", though both conditions have been observed in the same family.[1] This should not be confused with the germ line cri du chat (5p deletion) syndrome which is a deletion of the short arm of the 5th chromosome.[citation needed]
Symptoms and signs
The 5q-syndrome is characterized by macrocytic anemia, often a moderate thrombocytosis, erythroblastopenia, megakaryocyte hyperplasia with nuclear hypolobation, and an isolated interstitial deletion of chromosome 5. The 5q- syndrome is found predominantly in females of advanced age.[2]
Causes
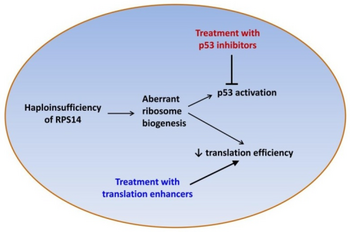
Several genes in the deleted region appear to play a role in the pathogenesis of 5q-syndrome.[3][4] Haploinsufficiency of RPS14 plays a central role, and contributes to the anemia via both p53-dependent and p53-independent tumor suppressor effects.[4] Other genes at this region include miR-145 and miR-146a, whose deletion is associated with the megakaryocytic dysplasia and thrombocytosis seen in 5q- syndrome;[5] SPARC, which has antiproliferative and antiangiogenic effects; and the candidate tumor suppressors EGR1, CTNNA1, and CDC25C.[4]
Histology
This syndrome affects bone marrow cells causing treatment-resistant anemia and myelodysplastic syndromes that may lead to acute myelogenous leukemia. Examination of the bone marrow shows characteristic changes in the megakaryocytes. They are more numerous than usual, small and mononuclear. There may be accompanying erythroid hypoplasia in the bone marrow.[6]
Diagnosis
The diagnosis of this condition is based on the following:[7]
- Medical history
- Medical exam
- Lab test
- Genetic test
Treatment
Lenalidomide has activity in 5q- syndrome[8] and is FDA approved for red blood cell (RBC) transfusion-dependent anemia due to low or intermediate-1 (int-1) risk myelodysplastic syndrome (MDS) associated with chromosome 5q deletion with or without additional cytogenetic abnormalities.[9]
There are several possible mechanisms that link the haploinsufficiency molecular lesions with lenalidomide sensitivity.[4][10]
Prognosis
Most affected people have a stable clinical course but are often transfusion dependent.[citation needed]
References
- ↑ Lazjuk GI; Lurie IW; Kirillova IA; et al. (August 1985). "Partial trisomy 5q and partial monosomy 5q within the same family". Clin. Genet. 28 (2): 122–9. doi:10.1111/j.1399-0004.1985.tb00371.x. PMID 4042393. S2CID 33815922.
- ↑ Naeim, Faramarz; Rao, P. Nagesh; W. Grody, Wayne (2008). Chapter 8 - Myelodysplastic Syndromes in Hematopathology Morphology, Immunophenotype, Cytogenetics and Molecular Approaches. pp. 129–154. doi:10.1016/B978-0-12-370607-2.00008-9.
- ↑ Ebert BL; Pretz J; Bosco J; et al. (January 2008). "Identification of RPS14 as a 5q- syndrome gene by RNA interference screen" (PDF). Nature. 451 (7176): 335–9. Bibcode:2008Natur.451..335E. doi:10.1038/nature06494. PMC 3771855. PMID 18202658. Archived (PDF) from the original on 2021-11-18. Retrieved 2021-11-20.
- ↑ 4.0 4.1 4.2 4.3 Nakhoul H, Ke J, Zhou X, et al. (2014). "Ribosomopathies: Mechanisms of disease". Clinical Medicine Insights: Blood Disorders. 7: 7–16. doi:10.4137/CMBD.S16952. PMC 4251057. PMID 25512719.
- ↑ Starczynowski DT; Kuchenbauer F; Argiropoulos B; et al. (January 2010). "Identification of miR-145 and miR-146a as mediators of the 5q- syndrome phenotype". Nature Medicine. 16 (1): 49–58. doi:10.1038/nm.2054. PMID 19898489. S2CID 7987486.
- ↑ Online Mendelian Inheritance in Man (OMIM): 5q- syndrome - 153550
- ↑ "5q- syndrome | Genetic and Rare Diseases Information Center (GARD) – an NCATS Program". rarediseases.info.nih.gov. Archived from the original on 21 November 2021. Retrieved 4 January 2022.
- ↑ List A; Dewald G; Bennett J; et al. (October 2006). "Lenalidomide in the myelodysplastic syndrome with chromosome 5q deletion". N. Engl. J. Med. 355 (14): 1456–65. doi:10.1056/NEJMoa061292. PMID 17021321.
- ↑ Raza, A; Reeves, J. A.; Feldman, E. J.; Dewald, G. W.; Bennett, J. M.; Deeg, H. J.; Dreisbach, L; Schiffer, C. A.; Stone, R. M.; Greenberg, P. L.; Curtin, P. T.; Klimek, V. M.; Shammo, J. M.; Thomas, D; Knight, R. D.; Schmidt, M; Wride, K; Zeldis, J. B.; List, A. F. (2008). "Phase 2 study of lenalidomide in transfusion-dependent, low-risk, and intermediate-1 risk myelodysplastic syndromes with karyotypes other than deletion 5q". Blood. 111 (1): 86–93. doi:10.1182/blood-2007-01-068833. PMID 17893227.
- ↑ Voutsadakis IA; Cairoli A (2012) (2012). "A critical review of the molecular pathophysiology of lenalidomide sensitivity in 5q – myelodysplastic syndromes". Leukemia & Lymphoma. 53 (5): 779–88. doi:10.3109/10428194.2011.623255. PMID 21955212. S2CID 38251104.
External links
- 5q- syndrome at NIH's Office of Rare Diseases
| Classification |
|---|
- Pages with script errors
- All articles with unsourced statements
- Articles with unsourced statements from November 2021
- Articles with invalid date parameter in template
- Articles with unsourced statements from July 2020
- Myeloid neoplasia
- Rare syndromes
- Autosomal monosomies and deletions
- Syndromes affecting blood