Meloxicam
 | |
 | |
| Names | |
|---|---|
| Trade names | Mobic, Metacam, Anjeso, others |
| |
| Clinical data | |
| Drug class | Nonsteroidal anti-inflammatory drug (NSAID) |
| Main uses | Pain, inflammation[1][2] |
| Side effects | Abdominal pain, dizziness, swelling, headache, rash[2] |
| Pregnancy category | |
| Routes of use | By mouth, IV |
| Defined daily dose | 15 mg[4] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a601242 |
| Legal | |
| License data | |
| Legal status | |
| Pharmacokinetics | |
| Bioavailability | 89%[5] |
| Protein binding | 99.4%[5] |
| Metabolism | Liver (CYP2C9 and 3A4-mediated)[5] |
| Elimination half-life | 20 hours[5] |
| Excretion | Urine and faeces equally[5] |
| Chemical and physical data | |
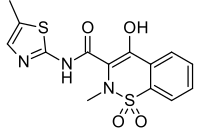
| Formula | C14H13N3O4S2 |
| Molar mass | 351.40 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Meloxicam, sold under the brand name Mobic among others, is a nonsteroidal anti-inflammatory drug (NSAID) used to treat pain and inflammation in rheumatic diseases and osteoarthritis.[1][2] It is used by mouth or by injection into a vein.[2][6] It is recommended that it be used for as short a period as possible and at a low dose.[2]
Common side effects include abdominal pain, dizziness, swelling, headache, and a rash.[2] Serious side effects may include heart disease, stroke, kidney problems, and stomach ulcers.[2] Use is not recommended in the third trimester of pregnancy.[2] It blocks cyclooxygenase-2 (COX-2) more than it blocks cyclooxygenase-1 (COX-1).[2] It is in the oxicam family of chemicals and is closely related to piroxicam.[2]
Meloxicam was patented in 1977 and approved for medical use in the United States in 2000.[2][7] It was developed by Boehringer Ingelheim, however it is also available as a generic medication.[2] In the United States the wholesale cost per dose is less than US$0.02 as of 2018[update].[8] In the United Kingdom it costs about 0.13 pounds as of 2018[update].[1] In 2017, it was the 38th most commonly prescribed medication in the United States, with more than 19 million prescriptions.[9][10]
Medical uses
Dosage
The defined daily dose is 15 mg by mouth, injection, or rectally.[4]
Side effects
Meloxicam use can result in gastrointestinal toxicity and bleeding, headaches, rash, and very dark or black stool (a sign of intestinal bleeding). Like other NSAIDs, its use is associated with an increased risk of cardiovascular events such as heart attack and stroke.[11] It has fewer gastrointestinal side effects than diclofenac,[12] piroxicam,[13] naproxen,[14] and perhaps all other NSAIDs which are not COX-2 selective.[12] Although meloxicam inhibits formation of thromboxane A, it does not appear to do so at levels that would interfere with platelet function.[medical citation needed]
A pooled analysis of randomized, controlled studies of meloxicam therapy of up to 60 days duration found that meloxicam was associated with a statistically significantly lower number of thromboembolic complications than the NSAID diclofenac (0.2% versus 0.8% respectively) but a similar incidence of thromboembolic events to naproxen and piroxicam.[15]
Cardiovascular
Persons with hypertension, high cholesterol, or diabetes are at risk for cardiovascular side effects. Persons with family history of heart disease, heart attack, or stroke must tell their treating physician as the potential for serious cardiovascular side effects is significant.[16][17]
Gastrointestinal
NSAIDs cause and increase the risk of serious gastrointestinal adverse events including bleeding, ulceration, and perforation of the stomach or intestines, which can be fatal. Elderly patients are at greater risk for serious gastrointestinal events.[18]
Mouth
It is recommended to withhold meloxicam use for at least four to six half-lives prior to surgical or dental procedures due to increased risk for taste perversion, ulcerative stomatitis and dry mouth.[medical citation needed]
Mechanism of action
Meloxicam blocks cyclooxygenase (COX), the enzyme responsible for converting arachidonic acid into prostaglandin H2—the first step in the synthesis of prostaglandins, which are mediators of inflammation. Meloxicam has been shown, especially at its low therapeutic doses, selectively to inhibit COX-2 over COX-1.[5]
Meloxicam concentrations in synovial fluid range from 40% to 50% of those in plasma. The free fraction in synovial fluid is 2.5 times higher than in plasma, due to the lower albumin content in synovial fluid as compared to plasma. The significance of this penetration is unknown,[18] but it may account for the fact that it performs exceptionally well in treatment of arthritis in animal models.[19]
Pharmacokinetics

Absorption
The bioavailability of meloxicam is decreased when administered orally compared to an equivalent IV bolus dose. Use of oral meloxicam following a high-fat breakfast increases the mean peak drug levels by about 22%; however, the manufacturer does not make any specific meal recommendations. In addition, the use of antacids does not show pharmacokinetic interactions.[21]
Distribution
The mean volume of distribution of meloxicam is approximately 10L. It is highly protein-bound, mainly to albumin.[medical citation needed]
Metabolism
Meloxicam is extensively metabolized in the liver by the enzymes CYP2C9 and CYP3A4 (minor) onto four inactive metabolites. Peroxidase activity is thought to be responsible for the other two remaining metabolites.[21][22]
Excretion
Meloxicam is predominantly excreted in the form of metabolites and occurs to equal extents in the urine and feces. Traces of unchanged parent drug are found in urine and feces. The mean elimination half-life ranges from 15 to 20 hours.[21]
Specific populations
Geriatrics
Use of meloxicam is not recommended in people with peptic ulcer disease or increased gastrointestinal bleed risk, including those over 75 years of age or those taking medications associated with bleeding risk.[23] Adverse events have been found to be dose-dependent and associated with length of treatment.[23][21]
Veterinary use
Meloxicam is used in veterinary medicine, most commonly in dogs and cats, but also sees off-label use in other animals such as cattle and exotics.[24][25]
Side effects in animals are similar to those found in humans; the principal side effect is gastrointestinal irritation (vomiting, diarrhea, and ulceration).[medical citation needed] Rarer but important side effects include liver and kidney toxicity.[medical citation needed]
In healthy dogs given meloxicam, no perioperative adverse effects on the cardiovascular system have been reported at recommended dosages.[26] Perioperative administration of meloxicam to cats did not affect postoperative respiratory rate nor heart rate.[27]
A peer-reviewed journal article cites NSAIDs, including meloxicam, as causing gastrointestinal upset and, at high doses, acute kidney injury and CNS signs such as seizures and comas in cats. It adds that cats have a low tolerance for NSAIDs.[28][29]
Meloxicam has been investigated as an alternative to diclofenac by the Royal Society for the Protection of Birds (RSPB) to prevent deaths of vultures.[30]
Pharmacokinetics
In dogs, the absorption of meloxicam from the stomach is not affected by the presence of food,[31] with the peak concentration (Cmax) of meloxicam occurring in the blood 7–8 hours after administration.[31] The half-life of meloxicam is approximately 24 hours in dogs.[31]
In the koala (Phascolarctos cinereus), very little meloxicam is absorbed into the blood after oral administration (that is, it has poor bioavailability).[32]
Legal status
United States
In 2003, meloxicam was approved in the U.S. for use in dogs for the management of pain and inflammation associated with osteoarthritis, as an oral (liquid) formulation of meloxicam.[33] In January 2005, the product insert added a warning in bold-face type: "Do not use in cats."[34] An injectable formulation for use in dogs was approved by the U.S. Food and Drug Administration (FDA) in November 2003.[35]
In October 2004, a formulation for use in cats was approved for use prior to surgery only.[36] This is an injectable meloxicam, indicated for as a single, one-time dose only, with specific and repeated warnings not to administer a second dose.[37]
In 2005, the U.S. Food and Drug Administration (FDA) sent a Notice of Violation to the manufacturer for its promotional materials which included promotion of the drug for off-label use.[38]
In February 2020, meloxicam injection was approved for use in the United States. The FDA granted the approval of Anjeso to Baudax Bio.[6][39]
European Union
In Europe, where the product has been available since the early 1990s,[citation needed] it is licensed for other anti-inflammatory benefits including relief from both acute and chronic pain in dogs. In June 2007, an oral version of meloxicam was licensed for the long-term relief of pain in cats.[40] Meloxicam is also licensed for use in horses, to relieve the pain associated with musculoskeletal disorders.[41]
Meloxicam was authorised for use in cattle throughout the European Union in January 1998, via a centralised marketing authorisation.[42] The first generic meloxicam product was approved in 2006.[42]
Other countries
As of June 2008[update], meloxicam is registered for long-term use in cats in Australia, New Zealand, and Canada.[40]
Society and culture
Cost
In the United States the wholesale cost per dose is less than US$0.02 as of 2018[update].[8] In the United Kingdom it costs about 0.13 pounds as of 2018[update].[1] In 2017, it was the 38th most commonly prescribed medication in the United States, with more than 19 million prescriptions.[9][10]
-
Meloxicam costs (US)
-
Meloxicam prescriptions (US)
References
- ↑ 1.0 1.1 1.2 1.3 British national formulary : BNF 76 (76 ed.). Pharmaceutical Press. 2018. pp. 1112–1113. ISBN 9780857113382.
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 "Meloxicam Monograph for Professionals". Drugs.com. AHFS. Archived from the original on 23 December 2018. Retrieved 23 December 2018.
- ↑ 3.0 3.1 Use During Pregnancy and Breastfeeding
- ↑ 4.0 4.1 "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 27 November 2020. Retrieved 9 September 2020.
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 Noble S, Balfour JA (March 1996). "Meloxicam". Drugs. 51 (3): 424–30, discussion 431–32. doi:10.2165/00003495-199651030-00007. PMID 8882380.
- ↑ 6.0 6.1 "Baudax Bio Announces FDA Approval of Anjeso for the Management of Moderate to Severe Pain". Baudax Bio, Inc. (Press release). 20 February 2020. Archived from the original on 21 February 2020. Retrieved 20 February 2020.
- ↑ Fischer, Jnos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 519. ISBN 9783527607495. Archived from the original on 10 July 2020. Retrieved 30 June 2020.
- ↑ 8.0 8.1 "NADAC as of 2018-12-19". Centers for Medicare & Medicaid Services (CMS). Archived from the original on 19 December 2018. Retrieved 22 December 2018.
- ↑ 9.0 9.1 "The Top 300 of 2020". ClinCalc. Archived from the original on 12 February 2021. Retrieved 11 April 2020.
- ↑ 10.0 10.1 "Meloxicam Drug Usage Statistics". ClinCalc. 23 December 2019. Archived from the original on 12 April 2020. Retrieved 11 April 2020.
- ↑ Stamm O, Latscha U, Janecek P, Campana A (January 1976). "Development of a special electrode for continuous subcutaneous pH measurement in the infant scalp". American Journal of Obstetrics and Gynecology. 124 (2): 193–5. doi:10.1016/S0002-9378(16)33297-5. PMID 2012.
- ↑ 12.0 12.1 Hawkey C, Kahan A, Steinbrück K, Alegre C, Baumelou E, Bégaud B, et al. (September 1998). "Gastrointestinal tolerability of meloxicam compared to diclofenac in osteoarthritis patients. International MELISSA Study Group. Meloxicam Large-scale International Study Safety Assessment". British Journal of Rheumatology. 37 (9): 937–45. doi:10.1093/rheumatology/37.9.937. PMID 9783757.
- ↑ Dequeker J, Hawkey C, Kahan A, Steinbrück K, Alegre C, Baumelou E, et al. (September 1998). "Improvement in gastrointestinal tolerability of the selective cyclooxygenase (COX)-2 inhibitor, meloxicam, compared with piroxicam: results of the Safety and Efficacy Large-scale Evaluation of COX-inhibiting Therapies (SELECT) trial in osteoarthritis". British Journal of Rheumatology. 37 (9): 946–51. doi:10.1093/rheumatology/37.9.946. PMID 9783758.
- ↑ Wojtulewski JA, Schattenkirchner M, Barceló P, Le Loët X, Bevis PJ, Bluhmki E, Distel M (April 1996). "A six-month double-blind trial to compare the efficacy and safety of meloxicam 7.5 mg daily and naproxen 750 mg daily in patients with rheumatoid arthritis". British Journal of Rheumatology. 35 Suppl 1: 22–8. doi:10.1093/rheumatology/35.suppl_1.22. PMID 8630632.
- ↑ Singh G, Lanes S, Triadafilopoulos G (July 2004). "Risk of serious upper gastrointestinal and cardiovascular thromboembolic complications with meloxicam". The American Journal of Medicine. 117 (2): 100–6. doi:10.1016/j.amjmed.2004.03.012. PMID 15234645.
- ↑ "Meloxicam". MedlinePlus. Archived from the original on 29 November 2014. Retrieved 15 November 2014.
- ↑ "Meloxicam". Drugs.com. Archived from the original on 16 November 2014. Retrieved 15 November 2014.
- ↑ 18.0 18.1 "Meloxicam official FDA information, side effects, and uses". Drugs.com. March 2010. Archived from the original on 16 March 2010. Retrieved 17 March 2010.
- ↑ Engelhardt G, Homma D, Schlegel K, Utzmann R, Schnitzler C (October 1995). "Anti-inflammatory, analgesic, antipyretic and related properties of meloxicam, a new non-steroidal anti-inflammatory agent with favourable gastrointestinal tolerance". Inflammation Research. 44 (10): 423–33. doi:10.1007/BF01757699. PMID 8564518. S2CID 37937305.
- ↑ "Meloxicam Pathway, Pharmacokinetics". PharmGKB. Retrieved 28 January 2024.
- ↑ 21.0 21.1 21.2 21.3 "Mobic- meloxicam tablet label". DailyMed. 11 October 2018. Archived from the original on 1 September 2020. Retrieved 2 August 2019.
- ↑ "Meloxicam (Professional Patient Advice)". Drugs.com. Archived from the original on 6 August 2019. Retrieved 6 August 2019.
- ↑ 23.0 23.1 2019 American Geriatrics Society Beers Criteria Update Expert Panel (April 2019). "American Geriatrics Society 2019 Updated AGS Beers Criteria® for Potentially Inappropriate Medication Use in Older Adults". Journal of the American Geriatrics Society. 67 (4): 674–694. doi:10.1111/jgs.15767. PMID 30693946. S2CID 59338182.
- ↑ Off-label use discussed in: Arnold Plotnick MS, DVM, ACVIM, ABVP, Pain Management using Metacam Archived 2011-07-14 at the Wayback Machine, and Stein, Robert, Perioperative Pain Management Archived 18 April 2010 at the Wayback Machine Part IV, Looking Beyond Butorphanol, Sep 2006, Veterinary Anesthesia & Analgesia Support Group.
- ↑ For off-label use example in rabbits, see Krempels, Dana, Hind Limb Paresis and Paralysis in Rabbits Archived 17 June 2010 at the Wayback Machine, University of Miami Biology Department.
- ↑ Boström IM, Nyman G, Hoppe A, Lord P (January 2006). "Effects of meloxicam on renal function in dogs with hypotension during anaesthesia". Veterinary Anaesthesia and Analgesia. 33 (1): 62–9. doi:10.1111/j.1467-2995.2005.00208.x. PMID 16412133.
- ↑ Höglund OV, Dyall B, Gräsman V, Edner A, Olsson U, Höglund K (October 2018). "Effect of non-steroidal anti-inflammatory drugs on postoperative respiratory and heart rate in cats subjected to ovariohysterectomy". Journal of Feline Medicine and Surgery. 20 (10): 980–984. doi:10.1177/1098612X17742290. PMID 29165006. S2CID 30649716.
- ↑ "Toxicology Brief: The 10 most common toxicoses in cats". Dvm360. 1 June 2006. Archived from the original on 29 August 2018. Retrieved 16 September 2018.
- ↑ Merola V, Dunayer E (June 2006). "The 10 most common toxicoses in cats" (PDF). Veterinary Medicine: 340–342. Archived (PDF) from the original on 9 August 2019. Retrieved 9 August 2019.
- ↑ Swan G, Naidoo V, Cuthbert R, Green RE, Pain DJ, Swarup D, et al. (March 2006). "Removing the threat of diclofenac to critically endangered Asian vultures". PLOS Biology. 4 (3): e66. doi:10.1371/journal.pbio.0040066. PMC 1351921. PMID 16435886.
- ↑ 31.0 31.1 31.2 Khan SA, McLean MK (March 2012). "Toxicology of frequently encountered nonsteroidal anti-inflammatory drugs in dogs and cats". The Veterinary Clinics of North America. Small Animal Practice. 42 (2): 289–306, vi–vii. doi:10.1016/j.cvsm.2012.01.003. PMID 22381180.
- ↑ Kimble B, Black LA, Li KM, Valtchev P, Gilchrist S, Gillett A, et al. (October 2013). "Pharmacokinetics of meloxicam in koalas (Phascolarctos cinereus) after intravenous, subcutaneous and oral administration". Journal of Veterinary Pharmacology and Therapeutics. 36 (5): 486–93. doi:10.1111/jvp.12038. PMID 23406022.
- ↑ "NADA 141-213: New Animal Drug Application Approval (for Metacam (meloxicam) 0.5 mg/mL and 1.5 mg/mL Oral Suspension)" (PDF). Food and Drug Administration (FDA). 15 April 2003. Archived from the original (PDF) on 6 April 2017. Retrieved 24 July 2010.
- ↑ "Client Information Sheet For Metacam (meloxicam) 1.5 mg/mL Oral Suspension" (PDF). Food and Drug Administration (FDA). January 2005. Archived from the original (PDF) on 15 November 2017.
Metacam is a prescription non-steroidal anti-inflammatory drug (NSAID) that is used to control pain and inflammation (soreness) due to osteoarthritis in dogs. Osteoarthritis (OA) is a painful condition caused by “wear and tear” of cartilage and other parts of the joints that may result in the following changes or signs in your dog: Limping or lameness, decreased activity or exercise (reluctance to stand, climb stairs, jump or run, or difficulty in performing these activities), stiffness or decreased movement of joints. Metacam is given to dogs by mouth. Do not use Metacam Oral Suspension in cats. Acute kidney injury and death have been associated with the use of meloxicam in cats.
- ↑ "NADA 141-219: Metacam (meloxicam) 5 mg/mL Solution for Injection" (PDF). U.S. Food and Drug Administration (FDA). 12 November 2003. Archived from the original (PDF) on 15 November 2017. Retrieved 8 August 2019.
- ↑ "Metacam 5 mg/mL Solution for Injection, Supplemental Approval" (PDF). U.S. Food and Drug Administration (FDA). 28 October 2004. Archived from the original (PDF) on 15 November 2017. Retrieved 8 August 2019.
- ↑ See the manufacturer's FAQ Archived 2 July 2011 at the Wayback Machine on its website, and its clinical dosing instructions for cats. Archived 2008-09-06 at the Wayback Machine
- ↑ "Notice of Violation" (PDF). U.S. Food and Drug Administration (FDA). 19 April 2005. Archived from the original (PDF) on 13 January 2017. Retrieved 8 August 2019.
- ↑ "Anjeso (meloxicam) injection, for intravenous use" (PDF). U.S. Food and Drug Administration (FDA). February 2020. Archived (PDF) from the original on 22 February 2020. Retrieved 21 February 2020.
- ↑ 40.0 40.1 Gaschen, Frederic P.; Schaer, Michael, eds. (2016). "Recent NSAID developments". Clinical medicine of the dog and cat (3rd ed.). CRC Press. ISBN 9781482226065. Archived from the original on 1 September 2020. Retrieved 28 January 2020.
- ↑ Maddison JE, Page SW, Church D, eds. (2008). "Meloxicam". Small animal clinical pharmacology (2nd ed.). Edinburgh: Saunders/Elsevier. pp. 301–302. ISBN 9780702028588.
- ↑ 42.0 42.1 Wright, Elizabethann (March 2007). "Generic and biosimilar medicinal products in the European Union" (PDF). Chemistry Today. 25 (2): 4–6. Archived (PDF) from the original on 28 January 2020. Retrieved 28 January 2020.
External links
| External sites: | |
|---|---|
| Identifiers: |
- Pages using duplicate arguments in template calls
- CS1: long volume value
- Webarchive template wayback links
- Use dmy dates from October 2019
- Articles with invalid date parameter in template
- Drugs with non-standard pregnancy category
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without UNII source
- Drugs missing an ATC code
- Drugboxes which contain changes to verified fields
- Drugboxes which contain changes to watched fields
- Articles containing potentially dated statements from 2018
- All articles containing potentially dated statements
- All articles with unsourced statements
- Articles with unsourced statements from July 2019
- Articles with unsourced statements from October 2012
- Articles containing potentially dated statements from June 2008
- Benzothiazines
- Boehringer Ingelheim
- Carboxamides
- Cat medications
- Dog medications
- Nonsteroidal anti-inflammatory drugs
- RTT
- Thiazoles
- Veterinary drugs

