Holoprosencephaly
| Holoprosencephaly | |
|---|---|
 | |
| Diagram depicting the main subdivisions of the embryonic vertebrate brain. | |
| Specialty | Medical genetics |
Holoprosencephaly (HPE) is a cephalic disorder in which the prosencephalon (the forebrain of the embryo) fails to develop into two hemispheres. Normally, the forebrain is formed and the face begins to develop in the fifth and sixth weeks of human pregnancy. The condition also occurs in other species.
The condition can be mild or severe. Most cases are not compatible with life and result in fetal death in utero.[1]
When the embryo's forebrain does not divide to form bilateral cerebral hemispheres (the left and right halves of the brain), it causes defects in the development of the face and in brain structure and function.
In less severe cases, babies are born with normal or near-normal brain development and facial deformities that may affect the eyes, nose, and upper lip.
Signs and symptoms
Symptoms of holoprosencephaly range from mild (no facial/organ defects, anosmia, or only a single central incisor) to moderate to severe (cyclopia).
-
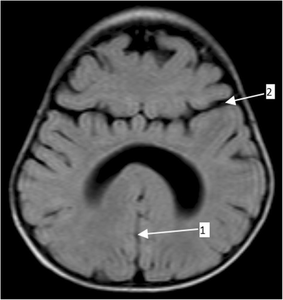
Semilobar holoprosencephaly-MRI shows incompletely interhemispheric fissure arrow 1 and partial fusion of frontal lobe arrow 2
-
Gross pathology specimen from a case of alobar holoprosencephaly.
There are four classifications of holoprosencephaly.
- Alobar holoprosencephaly, the most serious form, in which the brain fails to separate, is usually associated with severe facial anomalies, including lack of a nose and the eyes merged to a single median structure (see cyclopia).
- Semilobar holoprosencephaly, in which the brain's hemispheres have somewhat divided, is an intermediate form of the disease.
- Lobar holoprosencephaly, in which there is considerable evidence of separate brain hemispheres, is the least severe form. In some cases of lobar holoprosencephaly, the patient's brain may be nearly normal.
- Syntelencephaly, or middle interhemispheric variant of holoprosencephaly (MIHV), in which the posterior frontal lobe and the parietal lobe are not properly separated, but the rostro basal forebrain properly separates; it is possible that this is not a variant of HPE at all, but it is currently classified as such.[2]
- Agenesis of the corpus callosum, in which there is a complete or partial absence of the corpus callosum. It occurs when the corpus callosum, the band of white matter connecting the two hemispheres in the brain, fails to develop normally, typically during pregnancy. The fibers that would otherwise form the corpus callosum become longitudinally oriented within each hemisphere and form structures called Probst bundles.
Holoprosencephaly consists of a spectrum of defects or malformations of the brain and face. At the most severe end of this spectrum are cases involving serious malformations of the brain, malformations so severe that they often cause miscarriage or stillbirth. At the other end of the spectrum are individuals with facial defects which may affect the eyes, nose, and upper lip - and normal or near-normal brain development. Seizures and intellectual disabilities may occur.
The most severe of the facial defects (or anomalies) is cyclopia, an abnormality characterized by the development of a single eye, located in the area normally occupied by the root of the nose, and a missing nose or a nose in the form of a proboscis (a tubular appendage) located above the eye. The condition is also referred to as cyclocephaly or synophthalmia, and is very rare.
Causes
The exact cause(s) of HPE are yet to be determined. Mutations in the gene encoding the SHH protein, which is involved in the development of the central nervous system (CNS), can cause holoprosencephaly.[3][4][5] In other cases, it often seems that there is no specific cause at all.[6]
Genetics
Armand Marie Leroi describes the cause of cyclopia as a genetic malfunctioning during the process by which the embryonic brain is divided into two.[7] Only later does the visual cortex take recognizable form, and at this point an individual with a single forebrain region will be likely to have a single, possibly rather large, eye (at such a time, individuals with separate cerebral hemispheres would form two eyes).
Increases in expression of such genes as Pax-2, as well as inhibition of Pax-6, from the notochord have been implicated in normal differentiation of cephalic midline structures. Inappropriate expression of any of these genes may result in mild to severe forms of holoprosencephaly.[citation needed] Other candidate genes have been located, including the SHH (holoprosencephaly type 3 a.k.a. HPE3), TGIF, ZIC2, SIX3[8] and BOC genes.[9]
Although many children with holoprosencephaly have normal chromosomes, specific chromosomal abnormalities have been identified in some patients (trisomy of chromosome 13, also known as Patau syndrome). There is evidence that in some families, HPE is inherited (autosomal dominant as well as autosomal or X-linked recessive inheritance).[10][11][12] Features consistent with familial transmission of the disease (e.g., a single central maxillary incisor) should be carefully assessed in parents and family members.[13]
Non-genetic factors
Numerous possible risk factors have been identified, including gestational diabetes, transplacental infections (the "TORCH complex"), first trimester bleeding, and a history of miscarriage.[6][14] As well, the disorder is found twice as often in female babies.[14] However, there appears to be no correlation between HPE and maternal age.[14]
There is evidence of a correlation between HPE and the use of various drugs classified as being potentially unsafe for pregnant and lactating mothers. These include insulin, birth control pills, aspirin, lithium, thorazine, retinoic acid, and anticonvulsants.[14] There is also a correlation between alcohol consumption and HPE, along with nicotine, the toxins in cigarettes and toxins in cigarette smoke when used during pregnancy.[14]
Diagnosis

The diagnosis of this condition is done via the following methods:[15]
- MRI
- CT scan
- Ultrasound (prenatally)
Treatment
The management for this condition can be done via the following (though this is not an exhaustive list):[15]
- Supportive care
- Treatment for seizures
- Plastic reconstructive surgery
Prognosis
HPE is not a condition in which the brain deteriorates over time. Although serious seizure disorders, autonomic dysfunction, complicated endocrine disorders and other life-threatening conditions may sometimes be associated with HPE, the mere presence of HPE does not mean that these serious problems will occur or develop over time without any previous indication or warning. These abnormalities are usually recognized shortly after birth or early in life and only occur if areas of the brain controlling those functions are fused, malformed or absent.
Prognosis is dependent upon the degree of fusion and malformation of the brain, as well as other health complications that may be present.
The more severe forms of encephalopathy are usually fatal. This disorder consists of a spectrum of defects, malformations and associated abnormalities. Disability is based upon the degree in which the brain is affected. Moderate to severe defects may cause intellectual disability, spastic quadriparesis, athetoid movements, endocrine disorders, epilepsy and other serious conditions; mild brain defects may only cause learning or behavior problems with few motor impairments.
Seizures may develop over time with the highest risk before 2 years of age and the onset of puberty. Most are managed with one medication or a combination of medications. Typically, seizures that are difficult to control appear soon after birth, requiring more aggressive medication combinations/doses.
Most children with HPE are at risk of having elevated blood sodium levels during moderate-severe illnesses, that alter fluid intake/output, even if they have no previous diagnosis of diabetes insipidus or hypernatremia.
See also
References
- ↑ "Holoprosencephaly Information Page | National Institute of Neurological Disorders and Stroke". www.ninds.nih.gov. Archived from the original on 2021-08-13. Retrieved 2021-08-25.
- ↑ Totori-Donati P, Rossi A, Biancheri R (2005). "Brain Malformations". In Totori-Donati P, Rossi A, Raybaud C (eds.). Pediatric Neuroradiology: Brain, Head, Neck and Spine. Vol. 1. Springer. pp. 92–95. ISBN 978-3-540-41077-5.
- ↑ Chiang C, Litingtung Y, Lee E, Young KE, Corden JL, Westphal H, Beachy PA (October 1996). "Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function". Nature. 383 (6599): 407–13. Bibcode:1996Natur.383..407C. doi:10.1038/383407a0. PMID 8837770. S2CID 4339131.
- ↑ Muenke M, Beachy PA (June 2000). "Genetics of ventral forebrain development and holoprosencephaly". Current Opinion in Genetics & Development. 10 (3): 262–9. doi:10.1016/s0959-437x(00)00084-8. PMID 10826992. Archived from the original on 2021-11-04. Retrieved 2021-08-25.
- ↑ Rash BG, Grove EA (October 2007). "Patterning the dorsal telencephalon: a role for sonic hedgehog?". The Journal of Neuroscience. 27 (43): 11595–603. doi:10.1523/jneurosci.3204-07.2007. PMC 6673221. PMID 17959802.
- ↑ 6.0 6.1 The Carter Centers for Brain Research in Holoprosencephaly and Related Malformations. "About Holoprosencephaly". Archived from the original on 2009-05-14.
- ↑ Armand Marie Leroi, Mutants: On the Form, Varieties and Errors of the Human Body, 2003, Harper Perennial, London. ISBN 0-00-653164-4
- ↑ The Carter Center for Research in holoprosencephaly [1] Archived 2016-12-21 at the Wayback Machine and [2] Archived 2008-11-21 at the Wayback Machine
- ↑ Hong M, Srivastava K, Kim S, Allen BL, Leahy DJ, Hu P, Roessler E, Krauss RS, Muenke M (2017) BOC is a modifier gene in holoprosencephaly. Hum Mutat
- ↑ Singh S, Tokhunts R, Baubet V, Goetz JA, Huang ZJ, Schilling NS, et al. (February 2009). "Sonic hedgehog mutations identified in holoprosencephaly patients can act in a dominant negative manner". Human Genetics. 125 (1): 95–103. doi:10.1007/s00439-008-0599-0. PMC 2692056. PMID 19057928.
- ↑ Tekendo-Ngongang C, Muenke M, Kruszka P (1993). "Holoprosencephaly Overview". In Adam MP, Ardinger HH, Pagon RA, Wallace SE (eds.). GeneReviews®. Seattle (WA): University of Washington, Seattle. PMID 20301702. Archived from the original on 2020-11-27. Retrieved 2020-09-01.
- ↑ Nanni L, Ming JE, Bocian M, Steinhaus K, Bianchi DW, Die-Smulders C, et al. (December 1999). "The mutational spectrum of the sonic hedgehog gene in holoprosencephaly: SHH mutations cause a significant proportion of autosomal dominant holoprosencephaly". Human Molecular Genetics. 8 (13): 2479–88. doi:10.1093/hmg/8.13.2479. PMID 10556296.
- ↑ Nanni L, Ming JE, Du Y, Hall RK, Aldred M, Bankier A, Muenke M (July 2001). "SHH mutation is associated with solitary median maxillary central incisor: a study of 13 patients and review of the literature". American Journal of Medical Genetics. 102 (1): 1–10. doi:10.1002/1096-8628(20010722)102:1<1::aid-ajmg1336>3.0.co;2-u. PMID 11471164.
- ↑ 14.0 14.1 14.2 14.3 14.4 Croen LA, Shaw GM, Lammer EJ (February 2000). "Risk factors for cytogenetically normal holoprosencephaly in California: a population-based case-control study". American Journal of Medical Genetics. 90 (4): 320–5. doi:10.1002/(SICI)1096-8628(20000214)90:4<320::AID-AJMG11>3.0.CO;2-8. PMID 10710231.
- ↑ 15.0 15.1 "Holoprosencephaly". NORD (National Organization for Rare Disorders). Archived from the original on 21 March 2021. Retrieved 3 November 2021.
External links
| Classification | |
|---|---|
| External resources |
- GeneReview/NIH/UW entry on Holoprosencephaly Overview Archived 2020-11-27 at the Wayback Machine
- holoprosencephaly at NINDS
- What do we know about holoprosencephaly - Genome.gov Archived 2021-08-27 at the Wayback Machine