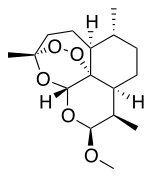
Artemether
 | |
 | |
| Names | |
|---|---|
| Trade names | Many[1] |
| |
| Clinical data | |
| Pregnancy category |
|
| Routes of use | Intramuscular[2] |
| Defined daily dose | 0.28 g (by mouth) 0.28 g (rectal) 0.12 g (parenteral)[3] |
| External links | |
| AHFS/Drugs.com | International Drug Names |
| Legal | |
| Legal status |
|
| Chemical and physical data | |
| Formula | C16H26O5 |
| Molar mass | 298.374 g/mol g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 86 to 88 °C (187 to 190 °F) |
| |
| |
Artemether is a medication used for the treatment of malaria.[2] The injectable form is specifically used for severe malaria rather than quinine.[2] In adults, it may not be as effective as artesunate.[2] It is given by injection in a muscle.[2] It is also available by mouth in combination with lumefantrine, known as artemether/lumefantrine.[4][5]
Artemether causes relatively few side effects.[6] An irregular heartbeat may rarely occur.[6] While there is evidence that use during pregnancy may be harmful in animals, there is no evidence of concern in humans.[6] The World Health Organization (WHO) therefore recommends its use during pregnancy.[6] It is in the artemisinin class of medication.[6]
Artemether has been studied since at least 1981, and been in medical use since 1987.[7] It is on the World Health Organization's List of Essential Medicines.[8] The wholesale cost in the developing world is between US$0.38 and US$16.47 per vial.[9] The combination form costs between US$100 and US$200 for a course of treatment in the United States.[10]
Medical uses
Artemether is an antimalarial drug for uncomplicated malaria caused by P. falciparum (and chloroquine-resistant P. falciparum) or chloroquine-resistant P. vivax parasites.[11] Artemether can also be used to treat severe malaria.[2]
The World Health Organization (WHO) recommends the treatment of uncomplicated P. falciparum with artemisinin-based combination therapy.[12] Given in combination with lumefantrine, it may be followed by a 14-day regimen of primaquine to prevent relapse of P. vivax or P. ovale malarial parasites and provide a complete cure.[13]
Artemether can also be used in treating and preventing trematode infections of schistosomiasis when used in combination with praziquantel.[14]
Artemether is rated category C by the FDA based on animal studies where artemisinin derivatives have shown an association with fetal loss and deformity. Some studies, however, do not show evidence of harm.[15][16]
Dosage
The defined daily dose is 0.28 g (by mouth) or 0.28 g(rectal) or 0.12 g (parenteral)[3]
Side effects
Possible side effects include cardiac effects such as bradycardia and QT interval prolongation.[17] Also, possible central nervous system toxicity has been shown in animal studies.[18][19]
Drug interactions
Plasma artemether level was found to be lower when the combination product was used with lopinavir/ritonavir.[19] There is also decreased drug exposure associated with concurrent use with efavirenz or nevirapine.[20][21]
Artemether/lumefantrine should not be used with drugs that inhibit CYP3A4.[22]
Hormonal contraceptives may not be as efficacious when used with artemether/lumefantrine.[22]
Pharmacology
Mechanism of action
Artemether is an artemisinin derivative and the mechanism of action for artemisinins is.[medical citation needed]
Artemether interact with ferriprotoporphyrin IX (heme) or ferrous ions in the acidic parasite food vacuole, and generates cytotoxic radical species[medical citation needed]
The accepted mode of action of the peroxide containing drug involve its interaction with heme (byproduct of hemoglobin degradation), derived from proteolysis of haemoglobin. This interaction results in the formation of toxic oxygen and carbon centered radicals.[medical citation needed]
One of the proposed mechanisms is that through inhibiting anti-oxidant and metabolic enzymes, artemisinin derivatives inflict oxidative and metabolic stress on the cell. Some pathways affected may concern glutathione and glucose metabolism. As a consequence, lesions and reduced growth of the parasite may result.[23]
Another possible mechanism of action suggests that arteristinin drugs exert their cidal action through inhibiting PfATP6. Since PfATP6 is an enzyme regulating cellular calcium concentration, its malfunctioning will lead to intracellular calcium accumulation, which in turns causes cell death.[24]
Pharmacokinetics
Absorption of artemether is improved 2- to 3-fold with food. It is highly bound to protein (95.4%). Peak concentrations of artemether are seen 2 hours after administration.[5]
Artemether is metabolized in the human body to the active metabolite, dihydroartemisinin, primarily by hepatic enzymes CYP3A4/5.[5] Both the parent drug and active metabolite are eliminated with a half-life of about 2 hours.[5]
Chemistry
Artemether is a methyl ether derivative of artemisinin, which is a peroxide-containing lactone isolated from the antimalarial plant Artemisia annua. It is also known as dihydroartemisinin methyl ether, but its correct chemical nomenclature is (+)-(3-alpha,5a-beta,6-beta,8a-beta, 9-alpha,12-beta,12aR)-decahydro-10-methoxy-3,6,9-trimethyl-3,12-epoxy-12H-pyrano(4,3-j)-1,2-benzodioxepin. It is a relatively lipophilic and unstable drug,[25] which acts by creating reactive free radicals in addition to affecting the membrane transport system of the plasmodium organism.[17]
References
- ↑ "Artemether - Drugs.com". www.drugs.com. Archived from the original on 20 December 2016. Retrieved 7 December 2016.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 Esu, Ekpereonne B.; Effa, Emmanuel E.; Opie, Oko N.; Meremikwu, Martin M. (18 June 2019). "Artemether for severe malaria". The Cochrane Database of Systematic Reviews. 6: CD010678. doi:10.1002/14651858.CD010678.pub3. ISSN 1469-493X. PMC 6580442. PMID 31210357.
- ↑ 3.0 3.1 "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 26 July 2020. Retrieved 21 September 2020.
- ↑ "Artemether and Lumefantrine". The American Society of Health-System Pharmacists. Archived from the original on 20 December 2016. Retrieved 28 November 2016.
- ↑ 5.0 5.1 5.2 5.3 "Coartem- artemether and lumefantrine tablet". DailyMed. 5 August 2019. Archived from the original on 26 July 2020. Retrieved 26 April 2020.
- ↑ 6.0 6.1 6.2 6.3 6.4 Kovacs, SD; Rijken, MJ; Stergachis, A (February 2015). "Treating severe malaria in pregnancy: a review of the evidence". Drug Safety. 38 (2): 165–81. doi:10.1007/s40264-014-0261-9. PMC 4328128. PMID 25556421.
- ↑ Rao, Yi; Zhang, Daqing; Li, Runhong (2016). Tu Youyou and the Discovery of Artemisinin: 2015 Nobel Laureate in Physiology or Medicine. World Scientific. p. 162. ISBN 9789813109919. Archived from the original on 2017-09-10.
- ↑ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ↑ "Artemether". International Drug Price Indicator Guide. Archived from the original on 22 January 2018. Retrieved 1 January 2016.
- ↑ Hamilton, Richart (2015). Tarascon Pocket Pharmacopoeia 2015 Deluxe Lab-Coat Edition. Jones & Bartlett Learning. p. 45. ISBN 9781284057560.
- ↑ Makanga, Michael; Krudsood, Srivicha (2009-10-12). "The clinical efficacy of artemether/lumefantrine (Coartem)". Malaria Journal. 8 (Suppl 1): S5. doi:10.1186/1475-2875-8-S1-S5. ISSN 1475-2875. PMC 2760240. PMID 19818172.
- ↑ Information, National Center for Biotechnology; Pike, U. S. National Library of Medicine 8600 Rockville; MD, Bethesda; Usa, 20894 (2015-01-01). TREATMENT OF UNCOMPLICATED PLASMODIUM FALCIPARUM MALARIA. World Health Organization. Archived from the original on 2017-09-10.
{{cite book}}: CS1 maint: numeric names: authors list (link) - ↑ Information, National Center for Biotechnology; Pike, U. S. National Library of Medicine 8600 Rockville; MD, Bethesda; Usa, 20894 (2015-01-01). Treatment Of Uncomplicated Malaria Caused By P. Vivax, P. Ovale, P. Malariae Or P. Knowlesi. World Health Organization. Archived from the original on 2017-09-10.
{{cite book}}: CS1 maint: numeric names: authors list (link) - ↑ Pérez del Villar, Luis; Burguillo, Francisco J.; López-Abán, Julio; Muro, Antonio (2012-01-01). "Systematic review and meta-analysis of artemisinin based therapies for the treatment and prevention of schistosomiasis". PLOS ONE. 7 (9): e45867. doi:10.1371/journal.pone.0045867. ISSN 1932-6203. PMC 3448694. PMID 23029285.
- ↑ Dellicour S, Hall S, Chandramohan D, Greenwood B (2007). "The safety of artemisinins during pregnancy: a pressing question". Malaria Journal. 6: 15. doi:10.1186/1475-2875-6-15. PMC 1802871. PMID 17300719.
- ↑ Piola P, Nabasumba C, Turyakira E, et al. (2010). "Efficacy and safety of artemether—lumefantrine compared with quinine in pregnant women with uncomplicated Plasmodium falciparum malaria: an open-label, randomised, non-inferiority trial". Lancet Infect Dis. 10 (11): 762–769. doi:10.1016/S1473-3099(10)70202-4. hdl:10144/116337. PMID 20932805.
- ↑ 17.0 17.1 "Artemether". www.antimicrobe.org. Archived from the original on 2017-02-23. Retrieved 2016-11-09.
- ↑ "WHO Model Prescribing Information: Drugs Used in Parasitic Diseases - Second Edition: Protozoa: Malaria: Artemether". apps.who.int. Archived from the original on 2016-11-10. Retrieved 2016-11-09.
- ↑ 19.0 19.1 Askling, Helena H.; Bruneel, Fabrice; Burchard, Gerd; Castelli, Francesco; Chiodini, Peter L.; Grobusch, Martin P.; Lopez-Vélez, Rogelio; Paul, Margaret; Petersen, Eskild (2012-01-01). "Management of imported malaria in Europe". Malaria Journal. 11: 328. doi:10.1186/1475-2875-11-328. ISSN 1475-2875. PMC 3489857. PMID 22985344.
- ↑ Van geertruyden, J.-P. (2014). "Interactions between malaria and human immunodeficiency virus anno 2014". Clinical Microbiology and Infection. 20 (4): 278–285. doi:10.1111/1469-0691.12597. PMC 4368411. PMID 24528518.
- ↑ Kiang, Tony K. L.; Wilby, Kyle J.; Ensom, Mary H. H. (2013-10-26). "Clinical Pharmacokinetic Drug Interactions Associated with Artemisinin Derivatives and HIV-Antivirals". Clinical Pharmacokinetics. 53 (2): 141–153. doi:10.1007/s40262-013-0110-5. ISSN 0312-5963. PMID 24158666.
- ↑ 22.0 22.1 Stover, Kayla R.; King, S. Travis; Robinson, Jessica (2012-04-01). "Artemether-Lumefantrine: An Option for Malaria". Annals of Pharmacotherapy. 46 (4): 567–577. doi:10.1345/aph.1Q539. ISSN 1060-0280. PMID 22496476.
- ↑ Saeed, ME; Krishna, S; Greten, HJ; Kremsner, PG; Efferth, T (August 2016). "Antischistosomal activity of artemisinin derivatives in vivo and in patients". Pharmacological Research. 110: 216–26. doi:10.1016/j.phrs.2016.02.017. PMID 26902577.
- ↑ Guo, Zongru (2016-03-01). "Artemisinin anti-malarial drugs in China". Acta Pharmaceutica Sinica B. 6 (2): 115–124. doi:10.1016/j.apsb.2016.01.008. PMC 4788711. PMID 27006895.
- ↑ De Spiegeleer, B.M.J.; D'Hondt, M.; Vangheluwe, E.; Vandercruyssen, K.; De Spiegeleer, B.G.I.; Jansen, H.; Koijen, I.; Van Gompel, J. (2012). "Relative response factor determination of artemether degradants with a dry heat stress approach". Journal of Pharmaceutical and Biomedical Analysis. 70: 111–116. doi:10.1016/j.jpba.2012.06.002. hdl:1854/LU-2938963. PMID 22770733. Archived from the original on 2019-03-29. Retrieved 2019-01-28.
== External links ==
| Identifiers: |
|
|---|
- Pages using duplicate arguments in template calls
- CS1 maint: numeric names: authors list
- Chem-molar-mass both hardcoded and calculated
- Infobox-drug molecular-weight unexpected-character
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without UNII source
- Drugs missing an ATC code
- Drugboxes which contain changes to verified fields
- Drugboxes which contain changes to watched fields
- All articles with unsourced statements
- Articles with unsourced statements from April 2020
- Articles with invalid date parameter in template
- Antimalarial agents
- Organic peroxides
- Sesquiterpenes
- Trioxanes
- Chinese discoveries
- World Health Organization essential medicines
- RTT
- Articles with changed CASNo identifier
- Articles with changed EBI identifier