Riboflavin deficiency
| Riboflavin deficiency | |
|---|---|
| Other names: Vitamin B2 deficiency | |
| Frequency | Lua error in Module:PrevalenceData at line 5: attempt to index field 'wikibase' (a nil value). |
Riboflavin deficiency can be due to inadequate intake, endocrine abnormalities and some diseases. The presentation can include skin disorders, angular stomatitis, itchy and red eyes. [1] In terms of the management multi-vitamin dietary supplements should be used[citation needed]
Signs and symptoms
Mild deficiencies can exceed 50% of the population in Third World countries and in refugee situations. Deficiency is uncommon in the United States and in other countries that have wheat flour, bread, pasta, corn meal or rice enrichment regulations. In the U.S., starting in the 1940s, flour, corn meal and rice have been fortified with B vitamins as a means of restoring some of what is lost in milling, bleaching and other processing. For adults 20 and older, average intake from food and beverages is 1.8 mg/day for women and 2.5 mg/day for men. An estimated 23% consume a riboflavin-containing dietary supplement that provides on average 10 mg. The U.S. Department of Health and Human Services conducts National Health and Nutrition Examination Survey every two years and reports food results in a series of reports referred to as "What We Eat In America." From NHANES 2011–2012, estimates were that 8% of women and 3% of men consumed less than the RDA. When compared to the lower Estimated Average Requirements, fewer than 3% did not achieve the EAR level.[citation needed]
Riboflavin deficiency (also called ariboflavinosis) results in stomatitis including painful red tongue with sore throat, chapped and fissured lips (cheilosis), and inflammation of the corners of the mouth (angular stomatitis). There can be oily scaly skin rashes on the scrotum, vulva, philtrum of the lip, or the nasolabial folds. The eyes can become itchy, watery, bloodshot and sensitive to light.[2] Due to interference with iron absorption, even mild to moderate riboflavin deficiency results in an anemia with normal cell size and normal hemoglobin content (i.e. normochromic normocytic anemia). This is distinct from anemia caused by deficiency of folic acid (B9) or cyanocobalamin (B12), which causes anemia with large blood cells (megaloblastic anemia).[3] Deficiency of riboflavin during pregnancy can result in birth defects including congenital heart defects[4] and limb deformities.[5] Prolonged riboflavin insufficiency is also known to cause degeneration of the liver and nervous system.[citation needed]
The stomatitis symptoms are similar to those seen in pellagra, which is caused by niacin (B3) deficiency. Therefore, riboflavin deficiency is sometimes called "pellagra sine pellagra" (pellagra without pellagra), because it causes stomatitis but not widespread peripheral skin lesions characteristic of niacin deficiency.[2]
Riboflavin deficiency prolongs recovery from malaria,[6] despite preventing growth of plasmodium (the malaria parasite).[7]
Causes
Riboflavin is continuously excreted in the urine of healthy individuals,[8] making deficiency relatively common when dietary intake is insufficient.[8] Riboflavin deficiency is usually found together with other nutrient deficiencies, particularly of other water-soluble vitamins. A deficiency of riboflavin can be primary – poor vitamin sources in one's daily diet – or secondary, which may be a result of conditions that affect absorption in the intestine, the body not being able to use the vitamin, or an increase in the excretion of the vitamin from the body.
Subclinical deficiency has also been observed in women taking oral contraceptives, in the elderly, in people with eating disorders, chronic alcoholism and in diseases such as HIV, inflammatory bowel disease, diabetes and chronic heart disease. The Celiac Disease Foundation points out that a gluten-free diet may be low in riboflavin (and other nutrients) as enriched wheat flour and wheat foods (bread, pasta, cereals, etc.) is a major dietary contribution to total riboflavin intake.[citation needed]
-
In terms of inborn errors of riboflavin transport three riboflavin transporters have been identified RFVT1 mostly expressed in small intestine, RFVT 2 mostly expressed in brain and RFVT 3 mostly expressed in small intestine[9]
-
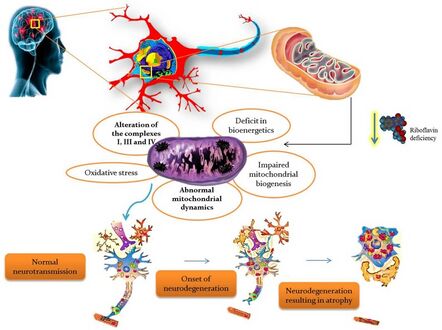
Riboflavin deficiency leading to mitochondrial oxidative stress-mediated neurodegeneration
Diagnosis
Overt clinical signs are rarely seen among inhabitants of the developed countries. The assessment of riboflavin status is essential for confirming cases with unspecific symptoms where deficiency is suspected.
- Glutathione reductase is a nicotinamide adenine dinucleotide phosphate (NADPH) and FAD-dependent enzyme, and the major flavoprotein in erythrocytes. The measurement of the activity coefficient of erythrocyte glutathione reductase (EGR) is the preferred method for assessing riboflavin status.[10] It provides a measure of tissue saturation and long-term riboflavin status. In vitro enzyme activity in terms of activity coefficients (AC) is determined both with and without the addition of FAD to the medium. ACs represent a ratio of the enzyme's activity with FAD to the enzyme's activity without FAD. An AC of 1.2 to 1.4, riboflavin status is considered low when FAD is added to stimulate enzyme activity. An AC > 1.4 suggests riboflavin deficiency. On the other hand, if FAD is added and AC is < 1.2, then riboflavin status is considered acceptable.[citation needed] Tillotson and Bashor[11] reported that a decrease in the intakes of riboflavin was associated with increase in EGR AC. In the UK study of Norwich elderly,[12] initial EGR AC values for both males and females were significantly correlated with those measured 2 years later, suggesting that EGR AC may be a reliable measure of long-term biochemical riboflavin status of individuals. These findings are consistent with earlier studies.[13]
- Experimental balance studies indicate that urinary riboflavin excretion rates increase slowly with increasing intakes, until intake level approach 1.0 mg/d, when tissue saturation occurs. At higher intakes, the rate of excretion increases dramatically.[10] Once intakes of 2.5 mg/d are reached, excretion becomes approximately equal to the rate of absorption[14] At such high intake a significant proportion of the riboflavin intake is not absorbed. If urinary riboflavin excretion is <19 µg/g creatinine (without recent riboflavin intake) or < 40 µg per day are indicative of deficiency.
Treatment
Multi-vitamin dietary supplements often contain 100% of the U.S. Daily Value (1.3 mg) for riboflavin, and can be used by persons concerned about an inadequate diet. Over-the-counter dietary supplements are available in the United States with doses as high as 100 mg, but there is no evidence that these high doses have any additional benefit for healthy people.[citation needed]
References
- ↑ "Office of Dietary Supplements - Riboflavin". ods.od.nih.gov. Archived from the original on 24 February 2020. Retrieved 8 May 2023.
- ↑ 2.0 2.1 Sebrell WH, Butler RE (1939). "Riboflavin Deficiency in Man (Ariboflavinosis)". Public Health Reports. 54 (48): 2121–2131. doi:10.2307/4583104. JSTOR 4583104.
- ↑ Lane M, Alfrey CP (Apr 1965). "The Anemia of Human Riboflavin Deficiency". Blood. 25 (4): 432–442. doi:10.1182/blood.V25.4.432.432. PMID 14284333.
- ↑ Smedts HP, Rakhshandehroo M, Verkleij-Hagoort AC, de Vries JH, Ottenkamp J, Steegers EA, Steegers-Theunissen RP (Oct 2008). "Maternal intake of fat, riboflavin and nicotinamide and the risk of having offspring with congenital heart defects". European Journal of Nutrition. 47 (7): 357–365. doi:10.1007/s00394-008-0735-6. PMID 18779918.
- ↑ Robitaille J, Carmichael SL, Shaw GM, Olney RS (Sep 2009). "Maternal nutrient intake and risks for transverse and longitudinal limb deficiencies: data from the National Birth Defects Prevention Study, 1997–2003". Birth Defects Research. Part A, Clinical and Molecular Teratology. 85 (9): 773–779. doi:10.1002/bdra.20587. PMID 19350655. Archived from the original on 13 June 2020. Retrieved 17 December 2019.
- ↑ Das BS, Das DB, Satpathy RN, Patnaik JK, Bose TK (April 1988). "Riboflavin deficiency and severity of malaria". European Journal of Clinical Nutrition. 42 (4): 277–83. PMID 3293996.
- ↑ Dutta P, Pinto J, Rivlin R (November 1985). "Antimalarial effects of riboflavin deficiency". Lancet. 2 (8463): 1040–3. doi:10.1016/S0140-6736(85)90909-2. PMID 2865519.
- ↑ 8.0 8.1 Brody, Tom (1999). Nutritional Biochemistry. San Diego: Academic Press. ISBN 978-0-12-134836-6. OCLC 162571066.
- ↑ Jaeger, Bregje; Bosch, Annet M. (July 2016). "Clinical presentation and outcome of riboflavin transporter deficiency: mini review after five years of experience". Journal of Inherited Metabolic Disease. 39 (4): 559–564. doi:10.1007/s10545-016-9924-2. ISSN 1573-2665. Archived from the original on 3 December 2022. Retrieved 8 May 2023.
- ↑ 10.0 10.1 Rosalind, Gibson S. (2005) "Riboflavin" in Principles of Nutritional Assessment, 2nd ed. Oxford University Press.
- ↑ Tillotson JA, Baker EM (April 1972). "An enzymatic measurement of the riboflavin status in man". The American Journal of Clinical Nutrition. 25 (4): 425–31. doi:10.1093/ajcn/25.4.425. PMID 4400882.
- ↑ Bailey AL, Maisey S, Southon S, Wright AJ, Finglas PM, Fulcher RA (February 1997). "Relationships between micronutrient intake and biochemical indicators of nutrient adequacy in a "free-living' elderly UK population". The British Journal of Nutrition. 77 (2): 225–42. doi:10.1079/BJN19970026. PMID 9135369.
- ↑ Rutishauser IH, Bates CJ, Paul AA, Black AE, Mandal AR, Patnaik BK (July 1979). "Long-term vitamin status and dietary intake of healthy elderly subjects. 1. Riboflavin". The British Journal of Nutrition. 42 (1): 33–42. doi:10.1079/BJN19790087. PMID 486392.
- ↑ Horwitt MK, Harvey CC, Hills OW, Liebert E (June 1950). "Correlation of urinary excretion of riboflavin with dietary intake and symptoms of ariboflavinosis". The Journal of Nutrition. 41 (2): 247–64. doi:10.1093/jn/41.2.247. PMID 15422413.
External links
| Classification |
|---|
![In terms of inborn errors of riboflavin transport three riboflavin transporters have been identified RFVT1 mostly expressed in small intestine, RFVT 2 mostly expressed in brain and RFVT 3 mostly expressed in small intestine[9]](https://nccommons.org/media/8/8e/PMC4920840_10545_2016_9924_Fig1_HTML.png)